| 年度報告2023 |
| 免責聲明PDF打印—本文件僅為"打印版",並非原始年度 財務報告,包括根據《荷蘭民法典》第2卷第361條的經審計財務報表。 這些原始年度財務報告包含在已審計財務報表和 審計師報告中,包含在單一報告包中,可在 https://www.example.com上找到 |
| 2023 Annual Report including the Annual Financial Statements for the year ended December 31, 2023 This Annual Report is filed with the Dutch Authority for the Financial Markets (Stichting Autoriteit Financiële Markten, AFM). The following main items included in our annual report on Form 20-F for the year ended December 31, 2023 (2023 20-F) filed with the United States Securities and Exchange Commission (SEC) on or about the date of this Annual Report have not been included in this Annual Report: · Form 20-F cover page; · Item 7 – Major Shareholders and Related Party Transactions; · Item 10E – Taxation; · Item 16E – Purchases of Equity Securities by the Issuer and Affiliated Purchasers · Item 16G – Corporate Governance; · Report of Independent Registered Public Accounting Firm in respect of Internal Control over Financial Reporting for the SEC filing; · Report of Independent Registered Public Accounting Firm in respect of the PCAOB audits of the 2023 financial statements for the SEC filing; · Exhibits; and · Signatures. The following main sections of our Annual Report have not been included in our 2023 20-F: · Shareholder Letter; · Outlook 2023; · Statement of the Board of Directors; · Risk Appetite and Control; · Share Classes and Principal Shareholders; · Non-Financial Information; · The Company Financial Statements under section Financial Statements (prepared pursuant to Dutch law); · Independent auditor’s report - Report on the audit of the financial statements 2023 included in the Annual Report with respect to the AFM Filing; and · Glossary. Certain defined terms argenx SE (herein argenx or the Company and, together with its subsidiaries, the Group, we or us) is a European public company (Societas Europaea) incorporated under the laws of the Netherlands with its statutory seat in Rotterdam, the Netherlands. It is publicly listed in Belgium and the United States of America (the U.S.). The applicable regulations with respect to public information and protection of investors, as well as the commitments we make to securities and market authorities, are described in this Annual Report. We own various trademark registrations and applications, and unregistered trademarks, including VYVGART®, VYVGART HYTRULO™, VYVDURA®, ARGENX™, ABDEG™, NHANCE™, argenx Annual Report 2023 | 3 |
| 簡單抗體™,ARGENXMEDHUB™和我們的公司標誌。本年度報告中出現的其他公司的商號、商標和服務標誌均為其各自所有者的財產。僅為方便起見,本 年度報告中的商標和商號可能不帶®和™符號,但此類引用不應被解釋為其各自所有者不會根據適用法律在最大程度上主張其權利的任何指示。我們不打算使用或展示其他 公司的商標和商品名稱,以暗示與任何其他公司的關係、代言或 贊助。 VYVGART®(Efgartigimod Alfa)(VYVGART)已在美國、日本、歐洲、英國(英國)、以色列、大陸中國(大陸中國)和加拿大獲得批准,用於靜脈治療泛發性重症肌無力(GMG)。我們現已將VYVGART在美國、歐盟幾個國家、日本、大陸中國(通過我們的合作伙伴再鼎醫藥(再鼎醫藥))、以色列(通過我們的美迪生製藥有限公司)和加拿大商業化。VYVGART皮下(SC)(efgartigimod alfa+hyaluridase qvfc)(VYVGART SC)已在美國獲批為VYVGART HYTRULO™(VYVGART HYTRULO),並在日本獲得批准為VYVURA®(VYVURA)。VYVGART SC也已在歐盟和英國獲得批准,用於治療GMG。我們現在已經在美國(以VYVGART HYTRULO)和德國實現了VYVGART SC的商業化。VYVGART SC的定價和報銷討論仍在多個其他國家/地區進行,包括在歐盟和日本(作為 VYVDURA)。 對於VYVGART和VYVGART SC,我們的目標是獲得進一步的批准,我們正在努力擴大其他司法管轄區的商業化。 如果未具體説明,本年度報告中對VYVGART的引用應理解為對VYVGART和/或VYVGART SC的引用,包括與美國和VYVDURA有關的VYVGART HYTRULO根據具體情況而定。 我們經審計的綜合財務報表的編制基礎 我們的經審計的綜合財務報表是根據國際會計準則委員會(IASB)發佈並被歐盟(EU)採納的國際財務報告準則(IFRS)編制的。因此,我們的 合併財務報表在本年度報告中以美元表示。除另有説明外,本年度報告中所有提及的“美元”、“美元”和“美元”均指美元,所有提及的“歐元”、“歐元”和“歐元”均指歐元。 在本年度報告中,提及的美國存托股份是指美國存托股份(ADS)或以美國存托股份為代表的普通股。視情況而定。 前瞻性陳述 本年度報告包含某些前瞻性陳述。前瞻性陳述 指截至本年度報告發布之日與歷史事實或事件或事實或事件無關的任何陳述,或基於我們管理層目前可獲得的信息而得出的基於我們管理層的信念和假設的任何陳述。前瞻性的 表述一般通過使用前瞻性詞彙來識別,如“預期”、“抱負”、“相信”、“可以”、“繼續”、“可能”、“估計”、“預期”、“希望”、“打算”、“旨在”、“期待”、“可能”、“可能”、“目標”、“計劃”、“潛在”、“項目”、“預測”、“尋求”, “應該”、“目標”、“將”或這些術語的其他變體或否定,或通過對戰略的討論,儘管並不是所有前瞻性表述都包含這些識別詞語。這些2023年年報|4 |
| statements relate to our future results of operations and financial positions, prospects, developments, business strategies, plans and our objectives for future operations, results of clinical trials and regulatory approvals, and are based on analyses or forecasts of future developments and estimates of amounts not yet determinable. These forward-looking statements represent the view of management only as of the date of this Annual Report, and we disclaim any obligation to update forward-looking statements, except as may be otherwise required by law. The forward-looking statements in this Annual Report involve known and unknown risks, uncertainties and other factors that could cause our actual future results, performance and achievements to differ materially from those forecasted or suggested herein. Forward-looking statements include, but are not limited to, statements about: · the initiation, timing, progress, development and results of clinical trials of our product candidates, including new indications, alternative dosing regimens and treatment modalities, including statements regarding when results or interim analysis of the clinical trials will be available or made public; · the expansion of our business, including the further development of our sales and marketing abilities and our Immunology Innovation Program (IIP), and the value of our pipeline; · the potential attributes and benefits of our products and product candidates, including new indications, alternative dosing regimens and treatment modalities, and their competitive position with respect to other alternative treatments; · our ability to advance product candidates into, and successfully complete, clinical trials; · our estimates of the number of patients who suffer from the diseases we are targeting and the number of patients that will enroll in our clinical trials; · the commercialization of our products and product candidates, including new indications, alternative dosing regimens and treatment modalities, if approved; · the anticipated timing of market authorizations of our products, including new indications, alternative dosing regimens and treatment modalities; · the anticipated pricing and reimbursement of our products and product candidates, if approved; · our plans to have various programs to help patients afford our products, including patient assistance and co-pay coupon programs for eligible patients; · the timing or likelihood of regulatory filings and decisions for any products and product candidates, including new indications, alternative dosing regimens and treatment modalities; · our ability to establish sales, marketing and distribution capabilities for any of our products and product candidates that achieve regulatory approval; · our regulatory strategy and our ability to establish and maintain manufacturing arrangements for our products and product candidates; · the scope and duration of protection, including any exclusivity period, we are able to establish and maintain for intellectual property rights covering our products and product candidates, platform and technology, including our intention to seek patent term extensions where available; · our estimates regarding expenses, future revenues, cash burn, capital requirements and our needs for additional financing; · our financial performance, including potential volatility in the price of our ordinary shares and ADSs; · the rate and degree of market acceptance of our products and product candidates, if approved; argenx Annual Report 2023 | 5 |
| · the potential benefits of our current collaborations, including the possibility to access partner technology platforms or capabilities; · our plans and ability to enter into collaborations for additional programs or product candidates; · our plans and ability to enter into new distribution partnerships; · the impact of government laws and regulations on our business; · our expectations with respect to the timing and amount of any dividends; · our plans regarding our supply chain, including our reliance on third parties, including CMOs; and · the implementation of our diversity, equity and inclusion policy, including our goal to further improve diversity on our board of directors (Board of Directors). These include changes in general economic and business conditions. You should refer to section “Risk Factors” of this Annual Report for a discussion of important factors that may cause our actual results to differ materially from those expressed or implied by our forward-looking statements. As a result of these factors, we cannot assure you that the forward-looking statements in this Annual Report will prove to be accurate. Furthermore, if our forward-looking statements prove to be inaccurate, the inaccuracy may be material. In light of the significant uncertainties in these forward-looking statements, you should not regard these statements as a representation or warranty by us or any other person that we will achieve our objectives and plans in any specified time frame or at all. We undertake no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law. You should read this Annual Report and the documents that we reference in this Annual Report and have filed as exhibits to the Annual Report completely and with the understanding that our actual future results may be materially different from what we expect. We qualify all of our forward-looking statements by these cautionary statements. Information regarding market and industry statistics contained in this Annual Report is included based on information available to us that we believe is accurate. Forecasts and other forward-looking information obtained from this available information is subject to the same qualifications and the additional uncertainties accompanying any estimates of future market size, revenue and market acceptance of products and services. In addition, statements that include “we believe” and similar statements reflect our beliefs and opinions on the relevant subject. These statements are based upon information available to us as of the date of this Annual Report, and while we believe such information forms a reasonable basis for such statements, such information may be limited or incomplete, and our statements should not be read to indicate that we have conducted an exhaustive inquiry into, or review of, all potentially available relevant information. These statements are inherently uncertain and you are cautioned not to unduly rely upon these statements. argenx Annual Report 2023 | 6 |
| 目錄 致股東 1集團的陳述 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 2風險因素 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 2.10 2.11股東函件11 2023簡介13 2024年展望24公司概況26戰略和目標30我們的產品和候選產品33合作和許可證54分銷協議63製造和供應63知識產權63法規67展示文件96摘要風險因素98與argenx財務狀況和需要額外資本有關的風險因素100與argenx產品和候選產品商業化有關的風險因素,新適應症包括102個與其他政府法規相關的風險因素111個與argenx產品和候選產品開發和臨牀試驗相關的風險因素118個與argenx相關的風險因素對第三方的依賴性123與argenx業務和行業相關的風險因素126與argenx知識產權相關的風險因素129與argenx組織和運營相關的風險因素135與ADS相關的風險因素139與外國私人發行人相關的風險因素 荷蘭公司141 argenx 2023年度報告|7 |
| 3企業管治 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 3.9 4公司概況及股本 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 4.10 4.11 4.12 4.13 4.14 4.15 4.16荷蘭公司治理守則145管理結構146非執行董事172薪酬報告及薪酬聲明176企業管治—納斯達克上市規則217股份所有權218內幕交易218網絡安全218風險偏好及控制權220本公司法律資料226股本227股份類別及主要股東232持有證券權利的限制236股東大會,投票權和入場236反收購條款239外匯管制239公司章程修正案239透明度指令239荷蘭財務報告監督法240股息及其他分配240在清算時獲得盈餘的權利241證券持有人權利的重大修改和 收益的使用242民事責任的執行242控制和程序244財務日曆245 argenx 2023年度報告|8 |
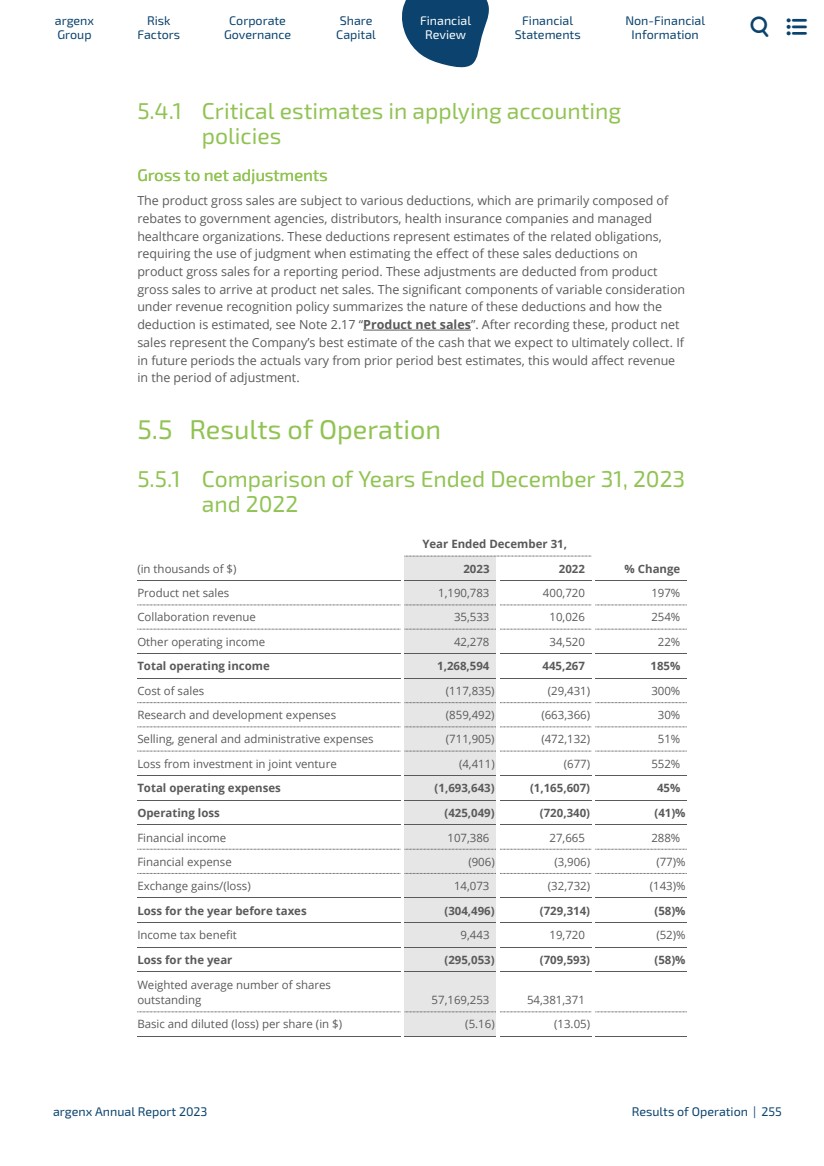
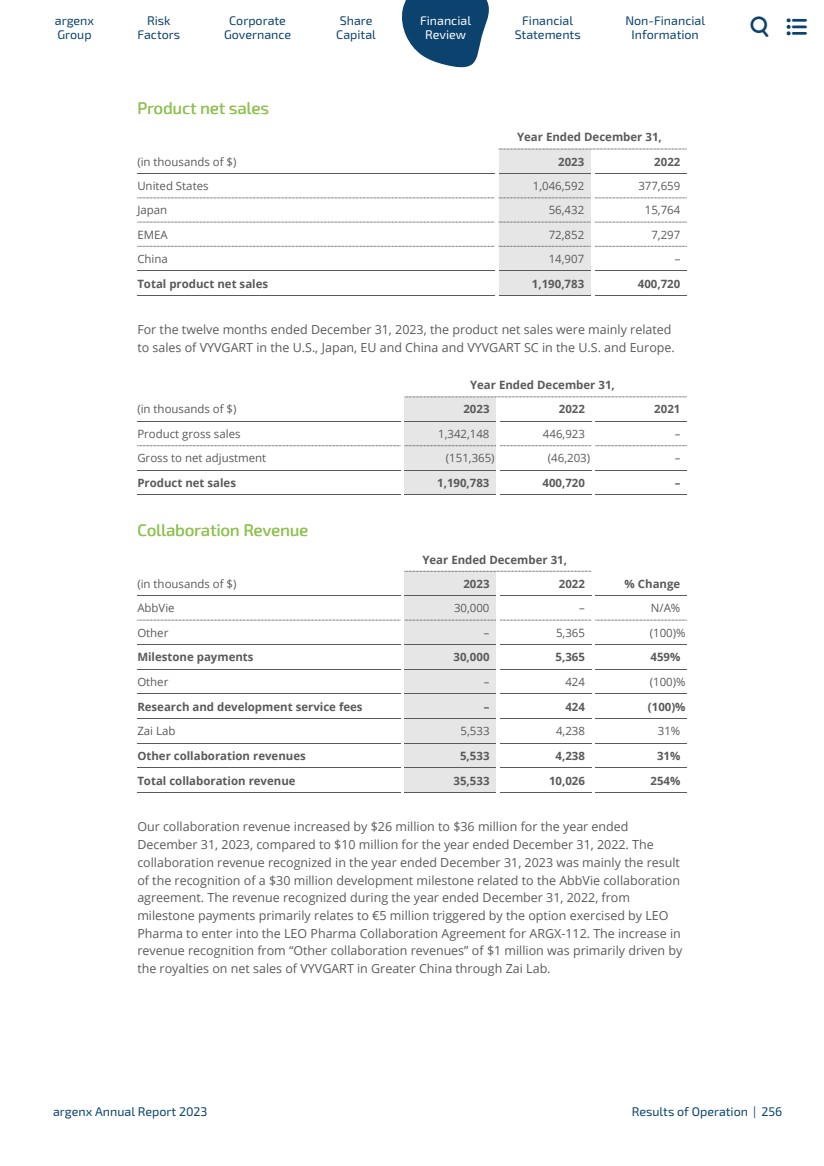
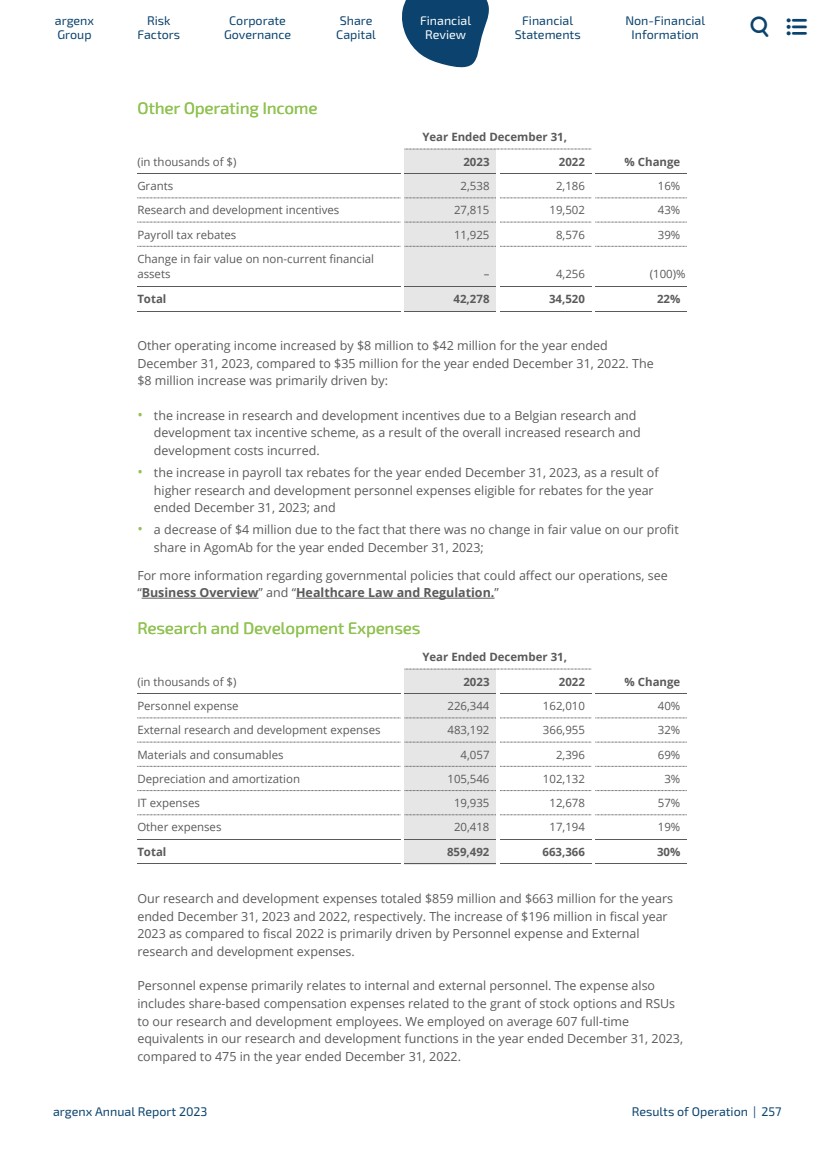
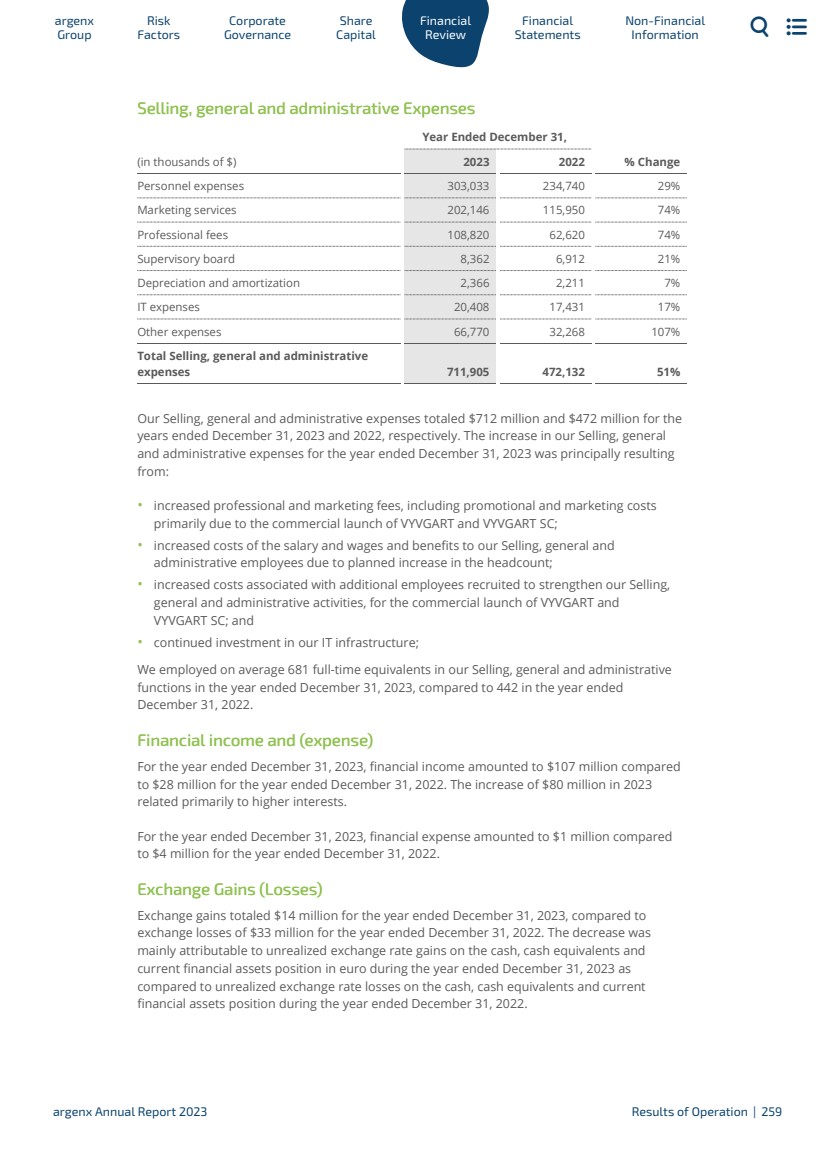
| 5運營和財務回顧與展望 5.1 5.2 5.3 5.4 5.5 5.6 5.7 5.8 5.9 5.10 5.11 5.12 5.13 5.14 5.15 5.16 6財務報表 6.1 6.2 6.3 7非財務信息 7.1 7.2 7.3 8術語表 8.1 8.2 8.3概覽247呈列基準249資本化和負債253關鍵會計估計和判斷254經營業績255流動性和資本資源260研究和開發,專利和許可證263趨勢信息264關閉—資產負債表安排264合同義務264有關獨立審計師的資料264重大合同及關聯方交易265僱員268保險268法律及仲裁訴訟程序269税務269合併財務報表291合併財務報表附註299 argenx SE截至12月31日止年度的公司財務報表,2023 344法規和合規363 NFRD 363歐盟分類372年度報告要求的交叉參考表382管理聲明383定義384 argenx 2023年度報告|9 |
| 致我們的 股東 股東函11 2023簡介13 2024展望24 argenx 2023年度報告致我們的股東|10 |
| Shareholder Letter Dear Shareholder, 2023 was a remarkable year for argenx as we carry forward our work to develop and deliver transformative therapies for autoimmune patients. We are strengthening and growing our ongoing collaborations with the world’s leading scientists to pioneer FcRn biology, while also developing novel molecules in the lab and clinic. And, as we execute on our ambitious business plan and step into our potential as a global organization, we hear more and more stories about the transformative impact VYVGART is having for patients, inspiring us to continue the work of rewriting the book on autoimmunity. We have now reached and improved the lives of over 6,000 gMG patients with VYVGART and this past year launched VYVGART HYTRULO, introducing optionality for patients and health care providers. VYVGART is setting new expectations in gMG with almost half of patients reaching minimal symptom expression. VYVGART has also shown meaningful steroid tapering, fast access to treatment and a very robust safety database. We were proud to earn more than $1.2 billion in revenue in 2023 and look forward to continued commercial excellence as we expand globally. Last summer, we shared groundbreaking results from our Phase 3 ADHERE clinical trial of VYVGART in chronic inflammatory demyelinating polyneuropathy (CIDP). In addition to providing a clinically meaningful benefit for patients and, importantly, a favorable safety profile, this clinical trial was of high quality and showed consistency across geographies. We have submitted our application for FDA approval, and if approved, look forward to launching mid-year. We know CIDP patients are waiting and we are eager to reach them with this life-changing treatment option which would represent the first real innovation for CIDP patients in many years. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Shareholder Letter | 11 |
| We are also advancing our second asset, empasiprubart, for which we achieved proof-of-concept (POC) in multifocal motor neuropathy (MMN). MMN patients often experience a lengthy, frustrating, and emotional diagnosis and lack effective treatment options. MMN patients are ready for a targeted and effective treatment option and we are committed to rapidly advancing this program. Looking ahead, we expect to file four new investigational new drug (IND) candidates by the end of 2025 delivered by our IIP. This program is a process innovation unique to argenx, and has been the driving force behind our work to pioneer first-in-class targets and is the engine that produced VYVGART, empasiprubart and ARGX-119 as well as partnered molecules such as ARGX-115 (AbbVie) and ARGX-112 (LEO Pharma). Our business model creates optionality within a molecule and within our pipeline bringing more first-in-class assets into clinical development. There will be attrition but the way you protect against it is by creating this type of optionality. We are driven by a relentless commitment to innovate for patients, but our ambition to innovate does not end in the lab. We are building argenx as a fully integrated and sustainable company – one where our people are inspired to grow our company, our partnerships, our science, and ourselves, because when we do, we deliver more for patients. We remain grateful for the continued support, encouragement, and advice from our investor community and collaborators, as together we forge ahead to pioneer novel biology that will bring new medicines to patients living with autoimmune disease. We will continue to execute on our strategy, with confidence in our products, pipeline, the people and the passion to achieve our bold ambition to transform autoimmunity. Sincerely, Tim Van Hauwermeiren & Peter Verhaeghe ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Shareholder Letter | 12 |
| 簡而言之,GMG只是我們轉變自身免疫的使命的開始。我們的第二個關鍵驅動因素是開創FcRN類藥物的先河,包括擴大我們正在使用efgartigimod評估的 適應症的範圍。截至2023年底,efgartigimod在三個適應症中獲得批准或正在接受監管審查,包括GMG、CIDP和原發免疫性血小板減少症(ITP),並正在 另外10多個嚴重的自身免疫適應症中進行評估。我們 正在很好地實現我們的‘argenx 2025’願景,即到2025年,efgartigimod將投入商業使用或臨牀 開發15種適應症。 第三,我們努力推進我們的差異化免疫資產管道。除了efgartigimod,我們全資擁有的臨牀流水線包括靶向補體成分2(C2)的Empasiprubart(ARGX-117) 和靶向肌肉特異性激酶(Musk)的ARGX-119。我們相信,這兩種療法都有潛力成為多種嚴重適應症的新型治療方式。 第四個關鍵驅動力是構建我們的創新生態系統,服務於我們的核心使命,即可持續地向需要的患者提供 免疫學創新。我們 繼續投資於我們的IIP,通過與正在研究一流疾病目標或 途徑的領先疾病生物學家合作,我們推動管道 的擴展。我們的IIP有着成功的記錄,自我們成立以來,已有9個 項目在人體上進行了測試。 在2023年初,我們分享了 年的關鍵驅動因素,以繼續我們的價值創造軌跡。首先, 我們的目標是在全球範圍內使用我們的一流新生兒Fc受體(FcRN)阻滯劑 VYVGART覆蓋更多的患者。VYVGART和VYVGART SC目前已在30多個國家或地區獲得批准 截至2023年底,我們已在全球範圍內用我們的創新治療了6,000多名GMG患者 。 |
| 覆蓋全球更多患者 VYVGART ·VYVGART現已在美國、日本、歐洲、英國、以色列、中國大陸 中國和加拿大獲得批准用於治療GMG。VYVGART SC現已在美國、歐洲、英國和日本獲得批准用於治療GMG。這使得VYVGART成為僅有的靜脈(IV)和簡單SC注射的GMG治療方法,為患者提供了選擇他們接受治療的方式和地點。2023年,我們產生了12億美元的產品淨銷售額 ·VYVGART和VYVGART SC的定價和報銷討論仍在多個司法管轄區進行 包括在歐盟的幾個國家 ·我們在日本申請批准VYVGART用於ITP,預計將在 2024年第一季度做出決定·針對SC efgartigimod的補充生物製品許可證申請(SBLA) CIDP已被FDA接受優先審查,處方藥用户費用法案(PDUFA)的目標日期為6月21日,2024年·我們已經在中國大陸申請批准VYVGART SC中國,我們預計在2024年底之前通過我們與再鼎醫藥的合作關係做出批准的決定 ·我們與韓國的Handok Inc.(Handok協議)簽訂了VYVGART商業和分銷協議(Handok協議) ·我們在幾個司法管轄區為GMG申請了VYVGART的批准,我們希望在2024年底之前做出多項決定。無限的符號 象徵着我們承諾 我們每年都會嘗試 為我們的患者 開發最佳解決方案,並推動我們向前邁進 Argenx年度報告2023年|簡要|14 |
| 先進的廣泛流水線 我們繼續展示我們免疫流水線的廣度和深度,並擁有 先進的多個產品中的流水線候選方案。有了efgartigimod,我們正在進一步鞏固我們在FcRN方面的領先地位,我們預計到2025年將在15種自身免疫性 適應症中獲得批准或正在開發中。除了efgartigimod,我們還在推進早期的流水線計劃,包括恩派西普魯巴特(C2抑制劑),正在進行MMN的POC第二階段臨牀試驗, 移植功能延遲(DGF)和皮肌炎(DM)。此外,我們預計將於2024年啟動馬斯克激動劑ARGX-119治療先天性肌無力綜合徵(CMS)和肌萎縮側索硬化症(ALS)的1b/2a期臨牀試驗。2023年Argenx年度報告 |
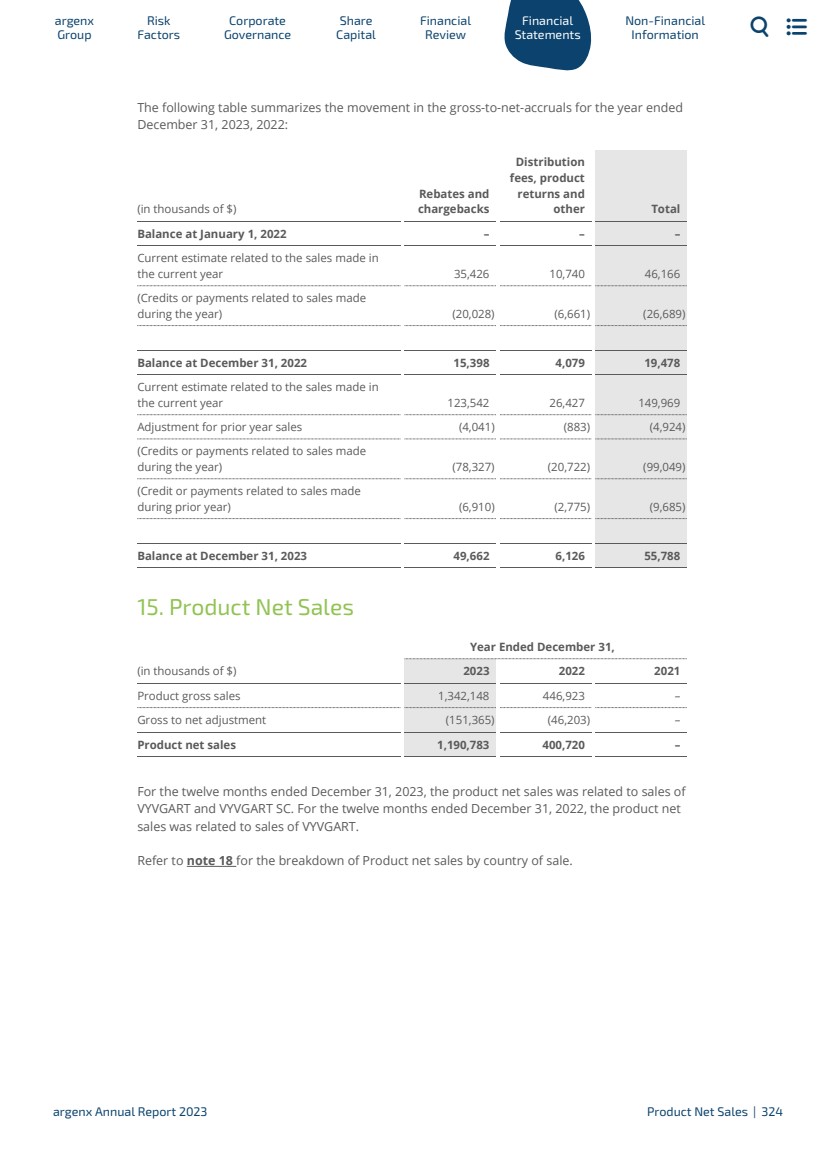
| 以Efgartigimod開創FcRN通路的先河 神經學適應症: ·依從性:根據CIDP的COND臨牀試驗的陽性背線結果,2023年12月21日提交了用於CIDP的SC Efgartigimod的sBLA,正在接受FDA的審查,PDUFA日期為2024年6月21日◦臨牀試驗滿足主要終點(p=0.000039);SC Efgartigimod 顯示減少了61%(HR:0.39 95%CI:0.25;0.61)復發風險與安慰劑 ·ALKIVIA:手術上無縫的2/3期臨牀試驗正在進行中,使用SC efgartigimod治療三種亞型的特發性炎症性肌病(肌炎),包括免疫介導性壞死性肌病(IMNM)、抗合成酶綜合徵(ASYS)和皮肌炎(DM);計劃對每個亞型的前30名患者進行分析 ·甲狀腺眼病的註冊臨牀試驗預計將於2024年第一季度開始血液學/風濕學適應症: ·Advance-IV:陽性臨牀試驗結果構成了在日本提交ITP的基礎; 2023年9月發表在《柳葉刀》上的TOPLINE結果·Advance-SC:2023年11月28日公佈的ITP中SC efgartigimod的TOPLINE數據未達到◦主要終點(p=0.5081);13.7%(17/124)的治療患者出現持續的血小板計數反應,而安慰劑患者的這一比例為16.2%(11/68)。次要終端也未滿足 argenx年度報告2023/2023簡要|16 |
| · RHO: Phase 2 POC clinical trial in primary Sjögren’s disease (SjD) is ongoing through our partnership with IQVIA Ltd (IQVIA) · ALPHA: Phase 2 POC clinical trial in post-COVID-19 postural orthostatic tachycardia syndrome (PC-POTS) ongoing through our partnership with IQVIA Dermatology indications: · ADDRESS: announced topline data of SC efgartigimod in pemphigus vulgaris (PV) and pemphigus foliaceus (PF) on December 20, 2023 ◦ Primary endpoint was not met; proportion of PV patients achieving primary endpoint of complete remission on minimal dose of steroids (CRmin) was not significantly different between SC efgartigimod and placebo ◦ Treatment with SC efgartigimod led to CRmin in 35.5% of patients compared to 30.3% with placebo (p=0.5956). Secondary endpoints were also not met · BALLAD: in light of ADDRESS results and the comparable biology between PV and bullous pemphigoid (BP), we decided to stop enrollment of BALLAD. We will integrate key learnings from ADDRESS and data from already-enrolled patients in BALLAD and we plan to communicate on a revised development plan before end of 2024 Nephrology indications: · Membranous Nephrology (MN): Phase 2 POC clinical trial ongoing through our partnership with Zai Lab · Lupus Nephritis (LN): Phase 2 POC clinical trial ongoing through our partnership with Zai Lab · Antibody-mediated rejection (AMR) Start of Phase 2 POC clinical trial is being prepared argenx Annual Report 2023 2023 In Brief | 17 |
| Broaden Immunology Pipeline with Empasiprubart and ARGX-119 Empasiprubart (C2 inhibitor): ARGX-119 (MuSK agonist): Build out Innovation Ecosystem · In January 2024, we announced the nomination of four new pipeline candidates, including: ARGX-213 targeting FcRn and furthering argenx’s leadership in this new class of medicine; ARGX-121 and ARGX-220, which are first-in-class targets broadening argenx’s focus across the immune system; and ARGX-109, targeting IL-6, which plays an important role in inflammation. Preclinical work is ongoing in each candidate. · We entered into a collaboration with Genmab A/S (Genmab) to jointly discover, develop and commercialize novel therapeutic antibodies with applications in immunology, as well as in oncology therapeutic areas. · ARDA: Phase 2 POC clinical trial ongoing of empasiprubart in MMN ◦ In January 2024, we reported positive clinical data from the first cohort of the Phase 2 POC ARDA clinical trial establishing POC in MMN. Empasiprubart demonstrated a 91% reduction in the need for intravenous Ig (IVIg) rescue compared to placebo [HR:0.09 95% CI(0.02;0.044)].安全性特徵 與I期數據一致 ·正在DGF和DM中進行的II期POC臨牀試驗 ·正在健康志願者中進行的I期劑量遞增臨牀試驗; 預計將於2024年開始的Ib/IIa期臨牀試驗,以評估CMS和ALS患者的早期信號檢測 argenx 2023年年度報告2023年簡介|18 |
| Corporate Achievements Steve Krognes Mr. Steve Krognes joined our Board of Directors in February 2023 as a non-executive director and chairperson of the audit and compliance committee J. Donald deBethizy Mr. J. Donald deBethizy, who has served as a director since May 2015, was appointed to serve as vice-chairman of the Board of Directors as of February 2023 Karen Massey Karen Massey joined argenx as chief operating officer (COO) in March 2023 succeeding Keith Woods. Mr. Woods transitioned to serve as strategic advisor to the commercialization committee of the Board of Directors 1,148 Employees Expansion to 1,148 full-time employees (as of December 31, 2023) to support further growth of our business, including fully staffed commercial teams in the U.S., Europe, Japan and Canada argenx Annual Report 2023 2023 In Brief | 19 |
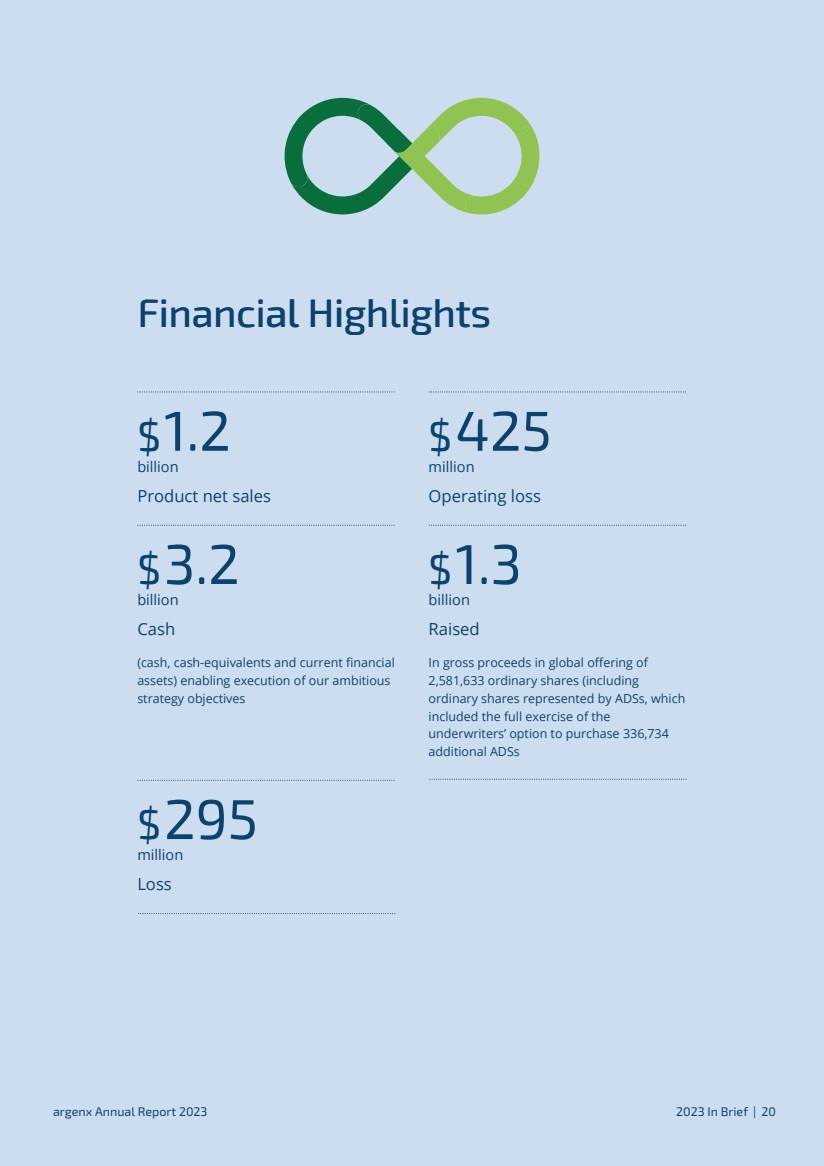
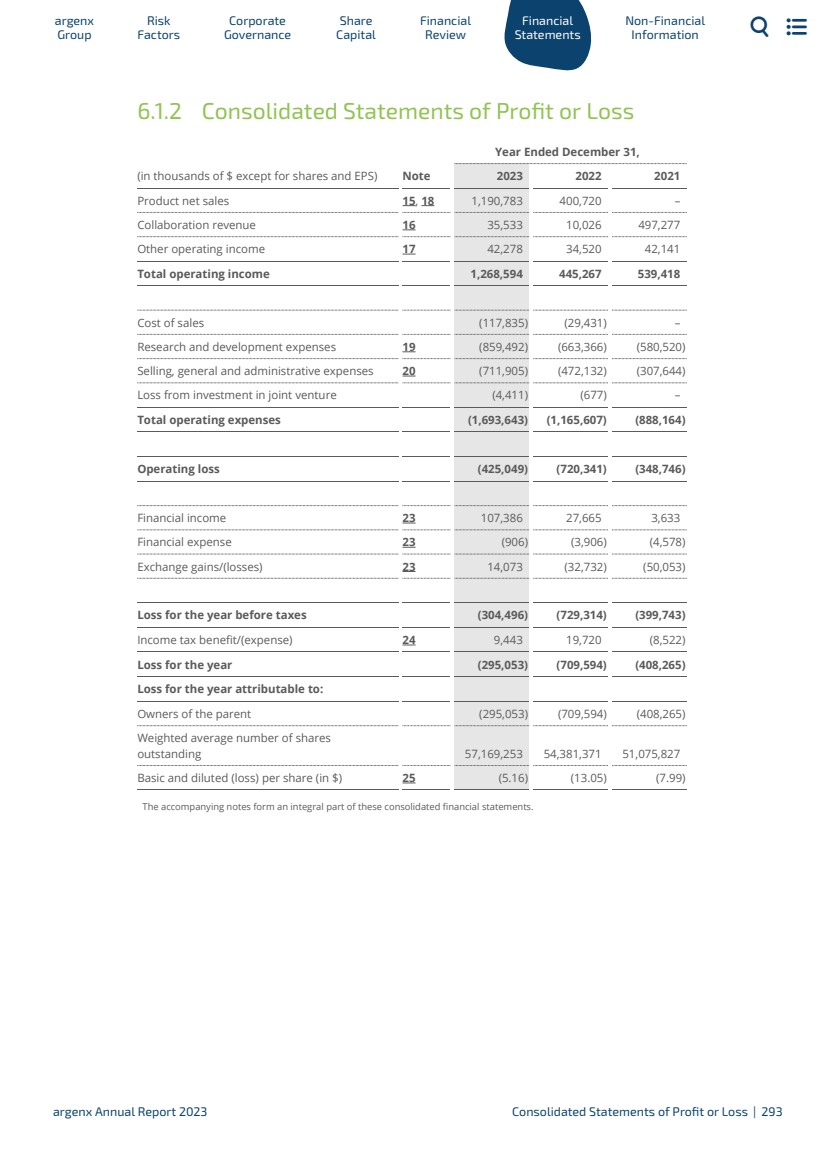
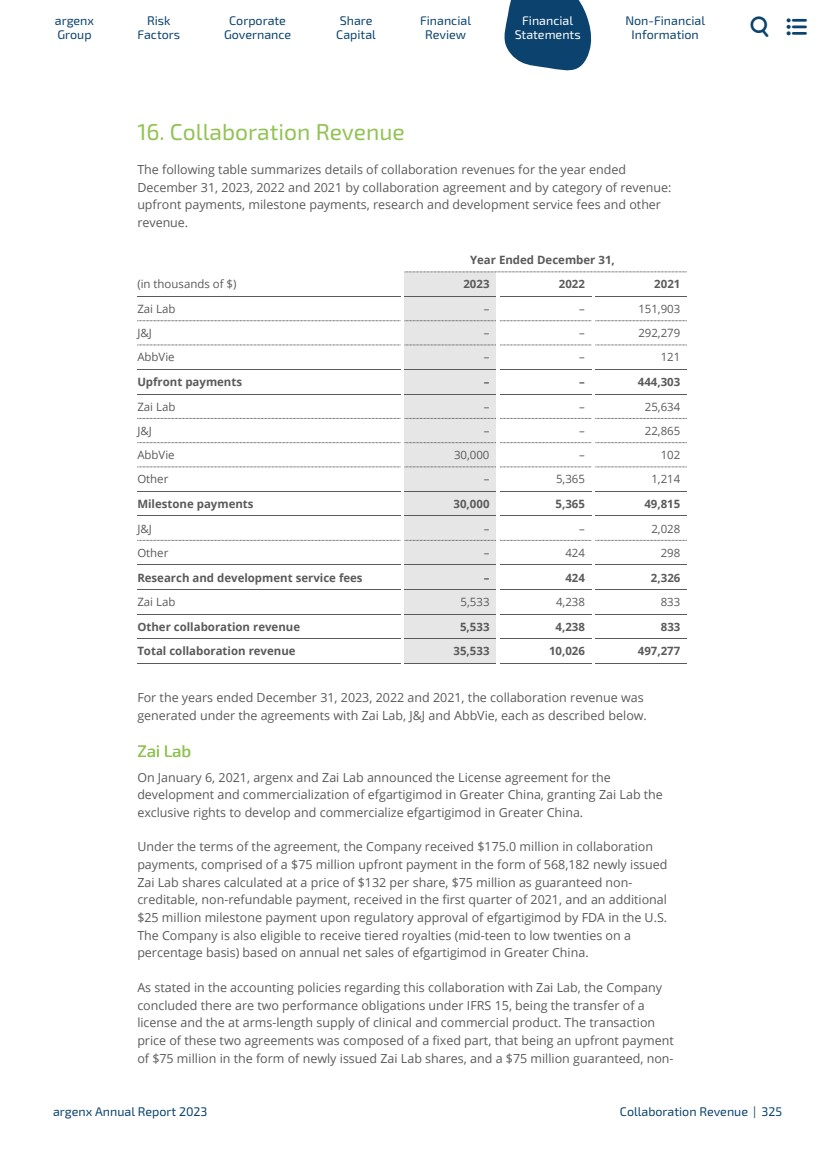
| Financial Highlights $1.2 billion Product net sales $425 million Operating loss $3.2 billion Cash (cash, cash-equivalents and current financial assets) enabling execution of our ambitious strategy objectives $1.3 billion Raised In gross proceeds in global offering of 2,581,633 ordinary shares (including ordinary shares represented by ADSs, which included the full exercise of the underwriters’ option to purchase 336,734 additional ADSs $295 million Loss argenx Annual Report 2023 2023 In Brief | 20 |
| 未來屬於 那些敢於 做得更多的人。 斯科特 "在很長一段時間裏,我害怕CIDP。然後有一天我對自己説,'你躲不掉它。 你必須讓它成為你自己的一部分 。 argenx 2023年度報告這是Scott的故事|21 |
| In the cab of his truck, Scott’s mind swirled as he frantically Googled CIDP for the first time. Scrolling through the definitions and symptoms of this rare condition, he felt frightened and overwhelmed. He had just left an appointment with the third neurologist he had seen in 2 years, who gave him a life-changing diagnosis and sent him out the door. Tears streamed down Scott’s face as the reality of living with a chronic illness set in, knowing his life was going to change. Scott, a dancer and choreographer, first started to notice symptoms while auditioning for a show in 2013. He was no stranger to hard work, but the tingling in his feet was something he had never felt before. He thought to himself, “Oh, I just put too much pressure on myself. This will go away.” Over the course of the show, Scott was on his feet a lot – dancing, directing, and choreographing – and the tingling remained. Concerned about complications from a previous hip replacement surgery, Scott reached out to his surgeon, who assured him that the tingling was not related to his hip procedure and suggested that he see a neurologist. Scott explained that he saw 2 different neurologists who weren’t familiar with CIDP at all. “CIDP is so rare that it wasn’t even on their radar.” Although his third neurologist was able to diagnose him with CIDP, he did not provide any support, so Scott sought better care. Once Scott got through the shock of his initial research into CIDP, Scott and his husband, Abel, set out to find out everything they could about CIDP. Fortunately, research led Scott to the GBS / CIDP Foundation International, where he learned of a CIDP Center of Excellence near his home. He now has a team of healthcare professionals to guide him. “I have had a team of 5 people taking care of me for the last 8 years. And it’s wonderful,” he said. CIDP Centers of Excellence are great sources for education and support. The turning point for Scott came when he realized that he needed to embrace his diagnosis in order to move forward. “For the longest time, I was scared of CIDP,” he thought. “Then one day I said to myself, ‘You can’t hide from it. You have to make it part of yourself.’” “Once I could say, ‘This is part of me. This is part of my life now. I have to deal with it. I have to grow with this thing,’ it made it OK.” Scott argenx Annual Report 2023 Scott’s story | 22 |
| “我曾經可以説,‘這是我的一部分。這是我現在生活的一部分。我必須要處理這件事。我必須 與這件事一起成長,‘它讓它變得沒問題。對Scott來説,擁抱CIDP不僅意味着承認他的病情嚴重,還意味着接受可以讓他的生活變得更輕鬆的事情 並讓他最親近的人提供幫助。他解釋説:“我推開了太多的東西,讓我的生活質量變得更好。如果這意味着輪椅或在我的車裏掛上殘疾標籤,我必須這麼做。“ 除了擁抱CIDP,Scott還專注於生活的三個方面: 管理他的CIDP: ·醫學方面--確保他聽醫生的話,完成每一次 治療預約 ·與他的護理團隊、家人、和朋友 ·克服CIDP診斷帶來的情緒壓力 “我必須學會如何繼續生活--每一天--即使對我來説事情將會有所不同。” 朋友和家人的支持也幫助Scott在日常生活中向前邁進 。“這些人和我一起經歷了情感的方方面面。我必須 記得説聲謝謝。斯科特解釋説。但斯科特也知道,與他的丈夫和照顧者亞伯進行適當的溝通是多麼重要,讓他知道自己每天的感受。斯科特説:“CIDP是一段每天的旅程;你可以有一天醒來感覺很強壯,也可以醒來後無法起牀。”他進一步解釋道:“你必須對照顧者非常詳細,否則他們幫不了你。 他們需要了解你在哪裏,這樣他們才能全天幫助你,因為你不想讓他們大吃一驚。”斯科特希望未來重返劇場,擔任董事和製片人,但他也意識到 他需要另一個表達自己的渠道。“每天都是你的新常態。這就像旋轉木馬,速度真的很快,你下不了車。我覺得我必須重塑自己,“他説。在這場鬥爭中,他靈光一閃,”我不知道是上帝還是宇宙,還是什麼東西砸在我頭上説,‘你要成為一名作家了。’“他意識到戲劇只是通過舞蹈和表演講故事的一種方式。寫作是另一個捕捉他必須分享的想法和故事的機會。 “我一生都想成為一名作家!我有很多想法都寫在紙上和筆記本上。他在寫作之前做了一些初步的研究,並閲讀了一些學術書籍,現在已經走上了正軌。 對於那些在CIDP社區尋找新激情的人,Scott提供了一些建議, “無論你的熱情是什麼,你都可能做不到。”但有一種方法可以重新創建 自己。我重新創造了一些關於我自己的東西,我真的對它們充滿了熱情。 |
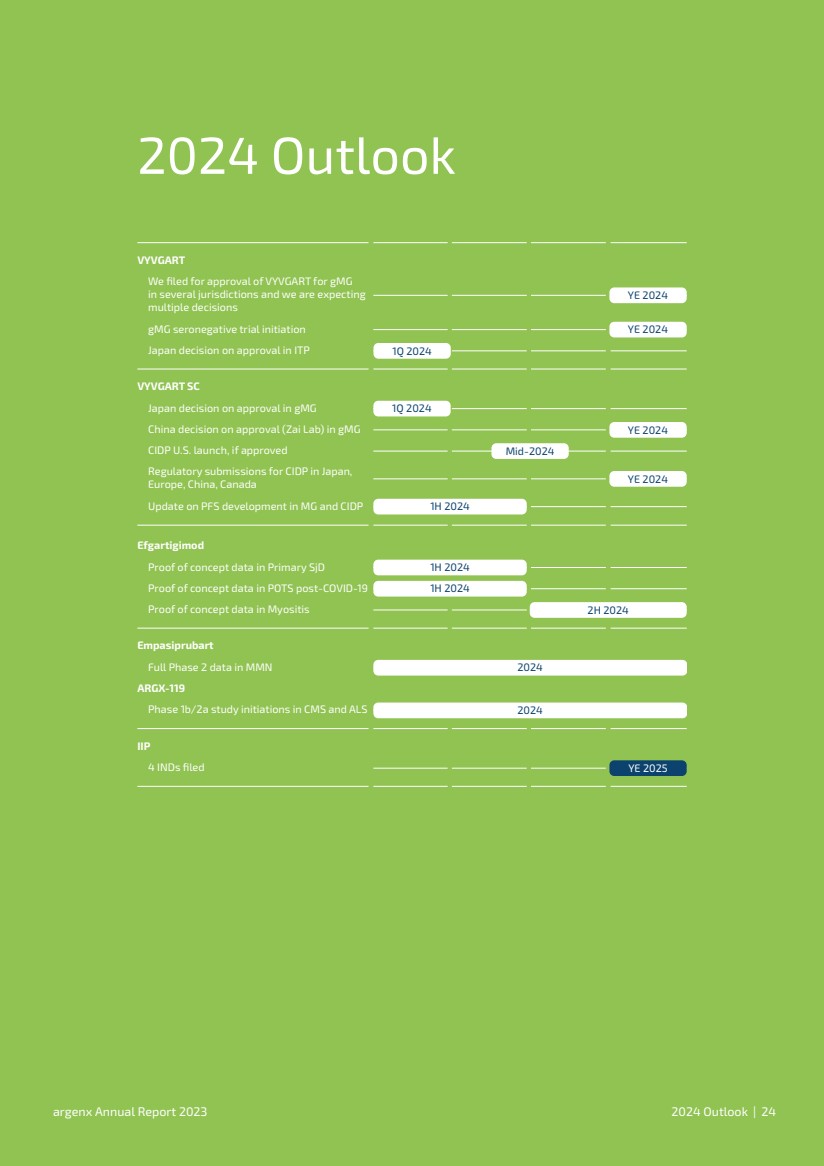
| 2024年展望 我們在多個司法管轄區申請批准GMG的VYVGART 我們預計將有 多項決定 GMG血清陰性試驗啟動 日本關於在ITP中批准的決定 VYVGART 日本關於在GMG中批准的決定 中國關於GMG中的批准(再鼎醫藥)的決定 如果獲得批准,CIMDP在日本、歐洲、中國、加拿大 MG和CIDP中PFS開發的更新 VYVGART SC 初級SJD中的概念驗證數據 新冠肺炎後POTS中的概念數據驗證Myositis中的概念驗證數據 Efgartigimod MMN中完整的第二階段數據 Empasiprubart CMS和ALS中的1b/2a階段研究啟動 IIP Ye 2024 Ye 2024 1Q 2024 1Q 2024 Mid-2024 2024 1H 2024 1H 2024 2H 2024 Ye 2024 Ye 2024 Ye 2025 Argenx年度報告2024 Ye 2024 Ye 2024 Ye 2025 Argenx年度報告2024 |
| 集團介紹 1.1公司簡介26 1.2戰略和目標30 1.3我們的產品和候選產品33 1.4合作和許可54 1.5分銷協議63 1.6製造和供應63 1.7知識產權63 1.8法規67 1.9展示的文件96 1 argenx年報2023 argenx集團|25 |
| 1 Presentation of the Group Company Profile General We are a commercial-stage, global, fully-integrated biopharma company developing a deep pipeline of differentiated therapies for the treatment of severe autoimmune diseases. By combining our suite of antibody engineering technologies with the disease biology expertise of our research collaborators, we aim to translate immunology breakthroughs into a pipeline of novel antibody-based medicines through our discovery engine, the IIP. We developed and are commercializing the first approved FcRn blocker in the U.S., Japan, Israel, the EU, Mainland China and Canada. We are evaluating efgartigimod in multiple serious autoimmune diseases and advancing several earlier stage experimental medicines. Our legal and commercial name is argenx SE. We were incorporated under the laws of the Netherlands on April 25, 2008, as a private company with limited liability (besloten vennootschap met beperkte aansprakelijkheid). From incorporation until August 28, 2009, our research and development activities were initially performed in the Netherlands, then Belgium, by argenx N.V. and its legal predecessors. Since August 28, 2009, all our research and development activities have been performed by our wholly-owned subsidiary, argenx BV, under a license provided by argenx N.V. Throughout this time, argenx BV assigned all resulting intellectual property to argenx N.V. On May 28, 2014, we converted to a Dutch public company with limited liability (naamloze vennootschap). On April 26, 2017, we converted to a Dutch European public company with limited liability (Societas Europaea or SE). On May 5, 2017, we transferred the legal ownership of all intellectual property rights of argenx SE to argenx BV, effective retroactively as of January 1, 2017. As a result, since January 1, 2017, (i) argenx BV holds all legal and economic ownership of our intellectual property rights, and (ii) the research and development agreement between argenx SE and argenx BV has been terminated. Our official seat is in Rotterdam, the Netherlands, and our registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. We are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our European legal entity identifier number (LEI) is 7245009C5FZE6G9ODQ71. Our telephone number is +31 (0) 10 70 38 441. Our website address is www.argenx.com. This website is not incorporated by reference in this Annual Report. The SEC maintains an Internet site that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC at www.sec.gov. The registered agent for service of process in the U.S. is CT Corporation System, with an address at 111 8th Avenue, New York, NY 10011. Our ordinary shares are listed on the regulated market of Euronext Brussels in Belgium under ISIN NL0010832176 under the symbol “ARGX” since 2014 and ADSs, each representing one ordinary share in argenx (or a right to receive such share), are listed on the Nasdaq Global Select Market (Nasdaq) under the symbol “ARGX” since 2017. 1.1 1.1.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Profile | 26 |
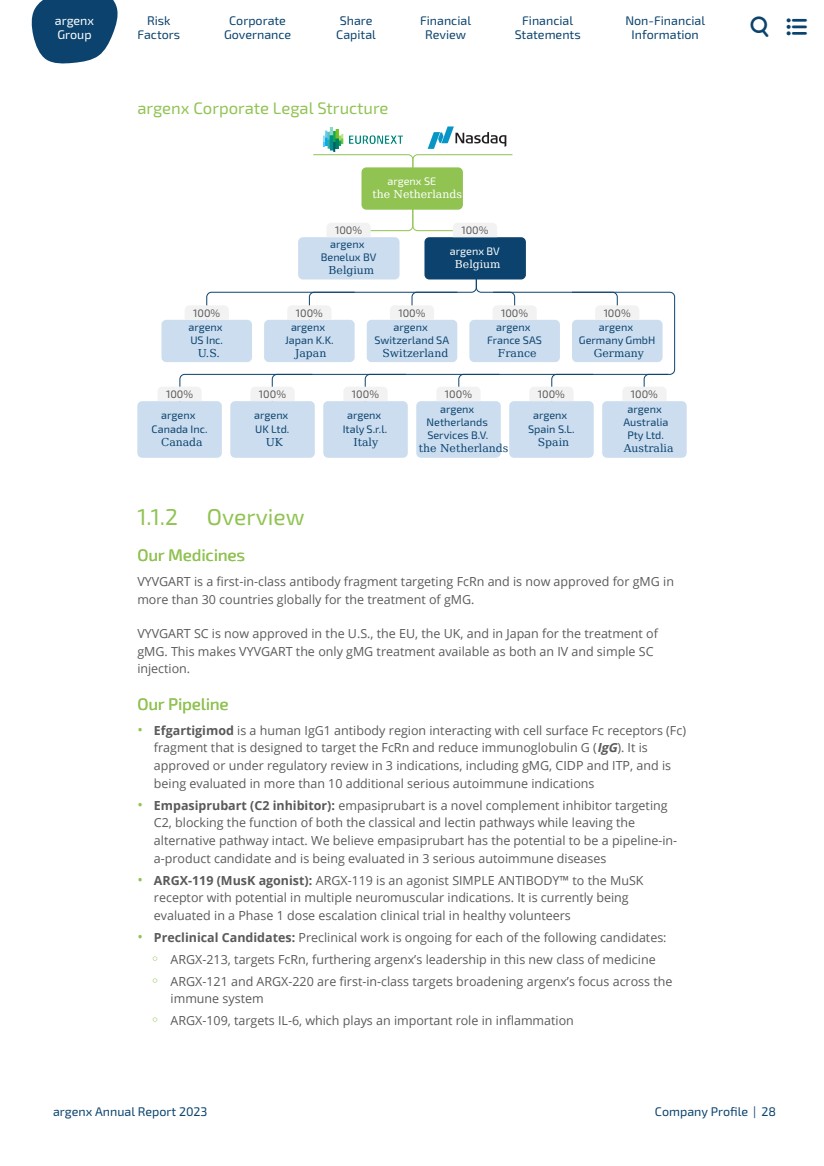
| argenx SE is the parent entity of the Group and the sole shareholder of: · argenx Benelux BV (prior to October 31, 2022 known as argenx IIP BV), a private company with limited liability (besloten vennootschap/société à responsabilité limitée) incorporated under the laws of Belgium, having its registered seat in Zwijnaarde, Belgium and its address at Industriepark-Zwijnaarde 7, 9052 Zwijnaarde, Belgium, and · argenx BV, a private company with limited liability (besloten vennootschap/société à responsabilité limitée) incorporated under the laws of Belgium, having its registered seat in Zwijnaarde, Belgium and its address at Industriepark-Zwijnaarde 7, 9052 Zwijnaarde, Belgium. argenx BV is the sole shareholder of: ◦ argenx US Inc., incorporated under the laws of the state of Delaware, U.S., having its registered office in Wilmington, Delaware and its address at 33 Arch Street, Boston, Massachusetts 02110; ◦ argenx Japan KK., incorporated under the laws of Japan, having its registered office in Tokyo, Japan and its address at HULIC JP Akasaka Building 2-5-8, Akasaka, Minato-ku, Tokyo, 107-0052, Japan; ◦ argenx Switzerland SA, incorporated under the laws of Switzerland, having its registered office in Geneva, Switzerland, and its address at Rue du Pré-de-la-Bichette 1, 1202 Geneva, Switzerland; ◦ argenx France SAS, incorporated under the laws of France, having its registered office in Paris, France, and its address at rue Camille Desmoulins 13, 92130 Issy Les Moulineaux, France; ◦ argenx UK Ltd., incorporated under the laws of the UK, having its registered office in Gerrards Cross, UK, and its address at Spaces Gerrards Cross Chalfont Park, Building 1 Gerrards Cross, SL9 0BG, UK; ◦ argenx Netherlands Services B.V., incorporated under the laws of the Netherlands, having its registered office in Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands; ◦ argenx Germany GmbH, incorporated under the laws of Germany, having its registered office in Munich, Germany, and its address at Konrad-Zuse-Platz 8, 81829 Munich, Germany; ◦ argenx Canada Inc., incorporated under the laws of Ontario, having its registered office in Ontario, Canada and its address at 9131 Keele Street Suite A4, Vaughan, Ontario, Canada, L4K 0G7; ◦ argenx Italy S.r.l., incorporated under the laws of Italy, having its registered office in Milan, Italy and its address at Largo Francesco Richini 6 CAP, 20122 Milan, Italy; ◦ argenx Spain S.L., incorporated under the laws of Spain, having its registered office in Madrid, Spain and its address at Paseo dela Castellana 200, Planta 8a, Oficina 819, 28046 Madrid, Spain; and ◦ argenx Australia Pty. Ltd., incorporated under the laws of Australia, having its registered office and address at Level 14, 2 Riverside Quay, Melbourne VIC 3006, Australia (since January 12, 2024). The following chart provides an overview of the Group as of the date of this Annual Report. Percentages refer to both the share of capital and voting rights. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Profile | 27 |
| argenx Corporate Legal Structure 100% 100% 100% 100% 100% 100% 100% 100% 100% 100% 100% 100% 100% argenx Spain S.L. Spain argenx Netherlands Services B.V. the Netherlands argenx Australia Pty Ltd. Australia argenx Switzerland SA Switzerland argenx Japan K.K. Japan argenx France SAS France argenx Germany GmbH Germany argenx US Inc. U.S. argenx UK Ltd. UK argenx Italy S.r.l. Italy argenx Canada Inc. Canada argenx Benelux BV Belgium argenx BV Belgium argenx SE the Netherlands Overview Our Medicines VYVGART is a first-in-class antibody fragment targeting FcRn and is now approved for gMG in more than 30 countries globally for the treatment of gMG. VYVGART SC is now approved in the U.S., the EU, the UK, and in Japan for the treatment of gMG. This makes VYVGART the only gMG treatment available as both an IV and simple SC injection. Our Pipeline · Efgartigimod is a human IgG1 antibody region interacting with cell surface Fc receptors (Fc) fragment that is designed to target the FcRn and reduce immunoglobulin G (IgG). It is approved or under regulatory review in 3 indications, including gMG, CIDP and ITP, and is being evaluated in more than 10 additional serious autoimmune indications · Empasiprubart (C2 inhibitor): empasiprubart is a novel complement inhibitor targeting C2, blocking the function of both the classical and lectin pathways while leaving the alternative pathway intact. We believe empasiprubart has the potential to be a pipeline-in-a-product candidate and is being evaluated in 3 serious autoimmune diseases · ARGX-119 (MusK agonist): ARGX-119 is an agonist SIMPLE ANTIBODY™ to the MuSK receptor with potential in multiple neuromuscular indications. It is currently being evaluated in a Phase 1 dose escalation clinical trial in healthy volunteers · Preclinical Candidates: Preclinical work is ongoing for each of the following candidates: ◦ ARGX-213, targets FcRn, furthering argenx’s leadership in this new class of medicine ◦ ARGX-121 and ARGX-220 are first-in-class targets broadening argenx’s focus across the immune system ◦ ARGX-109, targets IL-6, which plays an important role in inflammation 1.1.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Profile | 28 |
| · In addition to our wholly-owned pipeline, we have candidates that emerged from our IIP that we out-licensed to a partner for further development and for which we have milestone, royalty or profit-share agreements. These candidates include, amongst others: cusatuzumab (anti-CD70 antibody – Oncoverity), ARGX-112 (LP-0145 – anti-IL-22R antibody – LEO Pharma), ARGX-114 (AGMAB-101 – agonistic anti-MET antibody – Agomab) and ARGX-115 (ABBV-151 – anti-GARP antibody – AbbVie). IIP Our IIP is central to our core business strategy of co-creation and innovation. The IIP also serves as our discovery engine to identify novel targets and together, in collaboration with our scientific and academic partners, to build potential new pipeline candidates. Every current pipeline candidate from both our wholly-owned and partnered pipeline emerged from an IIP collaboration. The IIP enables us to build our broad pipeline of products and product candidates and advance our long-term strategy to be a sustainable, integrated immunology company. Examples of our IIP programs include: · Efgartigimod emerged from a collaboration with Professor Sally Ward at the University of Texas Southwestern Medical Center (UT Southwestern) and later became one of the blueprints for our IIP collaborations. Professor Ward’s research identified the crucial role that FcRn plays in maintaining and distributing IgGs throughout the body. Efgartigimod is a human IgG1 Fc fragment that is equipped with ABDEG™ mutations, which we in-licensed from UT Southwestern. These proprietary mutations modified efgartigimod to increase its affinity for FcRn while retaining the pH-dependent binding that is characteristic of FcRn interactions with its natural ligand, endogenous IgG. · Empasiprubart was built in collaboration with Broteio Pharma B.V. (Broteio). Broteio was launched in 2017 with support from Professor Erik Hack and the University of Utrecht, to conduct research demonstrating preclinical POC of the mechanism of action of empasiprubart. Professor Hack is a renowned researcher in the role of inflammation in disease, specifically in the complement system, and has contributed research and expertise to the approval of 2 complement inhibitors. His understanding of the mild phenotype associated with a natural C2 deficiency and C2’s unique positioning at the junction of the classical and lectin pathways led to our interest in engineering empasiprubart, with our proprietary NHANCE™ mutations and LALA mutations. · ARGX-119 was built in collaboration with the Leiden University Medical Center (LUMC) and New York University (NYU) with support from teams led by Professor Verschuuren and Professor Steve Burden, respectively. Both groups have world-class expertise in unraveling the biological mechanism of neuromuscular disease and translating these insights from the lab to the patient. Our Suite of Technologies · Through our IIP, we collaborate with scientific and academic partners to identify immunology breakthroughs and build potential pipeline candidates. This is done through co-creation. We bring to the collaboration our unique suite of antibody engineering technologies and experience in clinical development to complement our partners’ expertise in disease and target biology. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Profile | 29 |
| · SIMPLE ANTIBODY™ platform technology: Our proprietary SIMPLE ANTIBODY™ platform technology, based on the powerful llama immune system, allows us to exploit novel and complex disease biology targets. The platform sources antibody variable regions (V-regions) from the immune system of outbred llamas, each of which has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology, or similarity, in their V-regions when immunized with targets of human disease. Our SIMPLE ANTIBODY™ platform technology allows us to access and explore a broad target universe while potentially minimizing the long timelines associated with generating antibody candidates using traditional methods. · NHANCE™, ABDEG™, POTELLIGENT®, and DHS mutations focus on engineering the Fc region of antibodies in order to augment their intrinsic therapeutic properties. In addition, we obtained a non-exclusive research license and option from Chugai Pharmaceutical Co., Ltd. (Chugai) for the SMART-Ig® (‘Recycling Antibody’ and part of ‘Sweeping Antibody’) and ACT-Ig® (Antibody half-life extending) technologies. These technologies are designed to enable us to expand the therapeutic index of our product candidates, which is the ratio between toxic and therapeutic dose, by potentially modifying their half-life, tissue penetration, rate of disease target clearance and potency. In 2020, we also entered into a non-exclusive research agreement with the Clayton Foundation under which we may access the Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic antibodies. · Halozyme’s ENHANZE® SC drug delivery technology: we have exclusive access to ENHANZE® for FcRn, C2 and four additional target nominations. The global collaboration and license agreement with Halozyme Therapeutics, Inc. (Halozyme) was announced in February 2019 and expanded in October 2020. The ENHANZE® technology has the potential to shorten drug administration time, reduce healthcare practitioner time and offer additional flexibility and convenience for patients. · In April 2021, we entered into a collaboration and license agreement with Elektrofi, Inc. (Elektrofi) to explore Elektrofi’s high concentration technology for efgartigimod, and up to one additional target (Elektrofi Agreement). Strategy and Objectives Company’s Strategies Our goal is to deliver immunology innovations that are both first-in-class and best-in-class to transform the lives of people with serious autoimmune diseases. We do this by combining our leading antibody engineering capabilities with disease biology insights from our collaborators. Within this business model we plan to: · Continue to execute our global launch in gMG. One of our goals of 2023 was to expand our global launch of VYVGART as the first approved neonatal FcRn blocker for the treatment of gMG beyond initial commercial regions of the U.S., Japan and EU. In 2023, we received approval for VYVGART in Israel (through our partner Medison), the UK, Mainland China (through our partner Zai Lab) and Canada and we aim for further approvals in additional jurisdictions. We have built our commercial infrastructure to support the commercialization of VYVGART in the U.S., Europe, Japan and Canada and will be prepared to expand this infrastructure to support the launch of VYVGART into new indications in some of these territories if and when we receive approval. 1.2 1.2.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Strategy and Objectives | 30 |
| · Expand applications for our lead product efgartigimod beyond gMG. Our goal is to maximize the commercial potential of our existing products and product candidates by exploring additional indications, as well as formulations that may expand the target patient populations within existing indications. We are further developing our lead product, efgartigimod, for the treatment ofmore than 10 serious autoimmune diseases. We expand the use of our products and product candidates in existing indications by developing new formulations and product generations, that may reach more patient groups by capturing different patient preferences and providing additional optionality with regards to dosing. · Advance our pipeline of assets. In addition to new indications for efgartigimod, we plan to advance additional product candidates. In particular, we are advancing the clinical development of empasiprubart in MMN, DGF in the context of kidney transplants and DM. We are also advancing ARGX-119 into Phase 1b/2a clinical trials in CMS and ALS and beyond and plan to advance early-stage pipeline candidates towards IND filing by the end of 2025, as well as expand our pipeline of future product candidates through the IIP. · Leverage our suite of technologies to seek strategic collaborations and maximize the value of our pipeline. Our suite of technologies and productive discovery capabilities have yielded several potential product candidates for which we seek to capture value, while maintaining our focus and discipline. We plan to collaborate on product candidates that we believe have promising utility in disease areas or patient populations but fall outside our commercial franchises or are better served with the focus of a dedicated team in a spin-off company. In addition to collaborating on our products and product candidates, we may also elect to enter into collaborations for access to partner technology platforms or capabilities from which we can develop differentiated potential pipeline assets. · Continue to build innovation into every step of our development, highlighted by our collaborative IIP translating immunology breakthroughs into medicines. Our IIP is our core business strategy connecting the specialized insight into disease- and target biology of our external scientific and academic collaborators with our unparalleled experience as antibody engineers. Co-creation has led to a deep pipeline of highly differentiated product candidates. Through our IIP, we hope to together transcend breakthrough research and publications to our ultimate and unifying mission of creating new potential treatment options for patients. Trends Other than as disclosed elsewhere in this Annual Report, we are not aware of any trends, uncertainties, demands, commitments or events for the current financial period that are reasonably likely to have a material effect on our net revenues, income, profitability, liquidity, capital resources or prospects, or that caused the disclosed financial information to be not necessarily indicative of future operating results or financial conditions. Following the approval of VYVGART and VYVGART HYTRULO for the treatment of gMG in the U.S. by the FDA in 2021 and 2023 respectively, we transitioned from a clinical-stage to a commercial-stage biotechnology company. We have now commercialized VYVGART in U.S., the EU, Japan Mainland China (through our partner Zai Lab), Israel (through our partner Medison) and Canada, and VYVGART SC in the U.S. and Germany. We are working to expand commercialization in other jurisdictions, and to launch new products and product candidates, including into new indications. 1.2.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Strategy and Objectives | 31 |
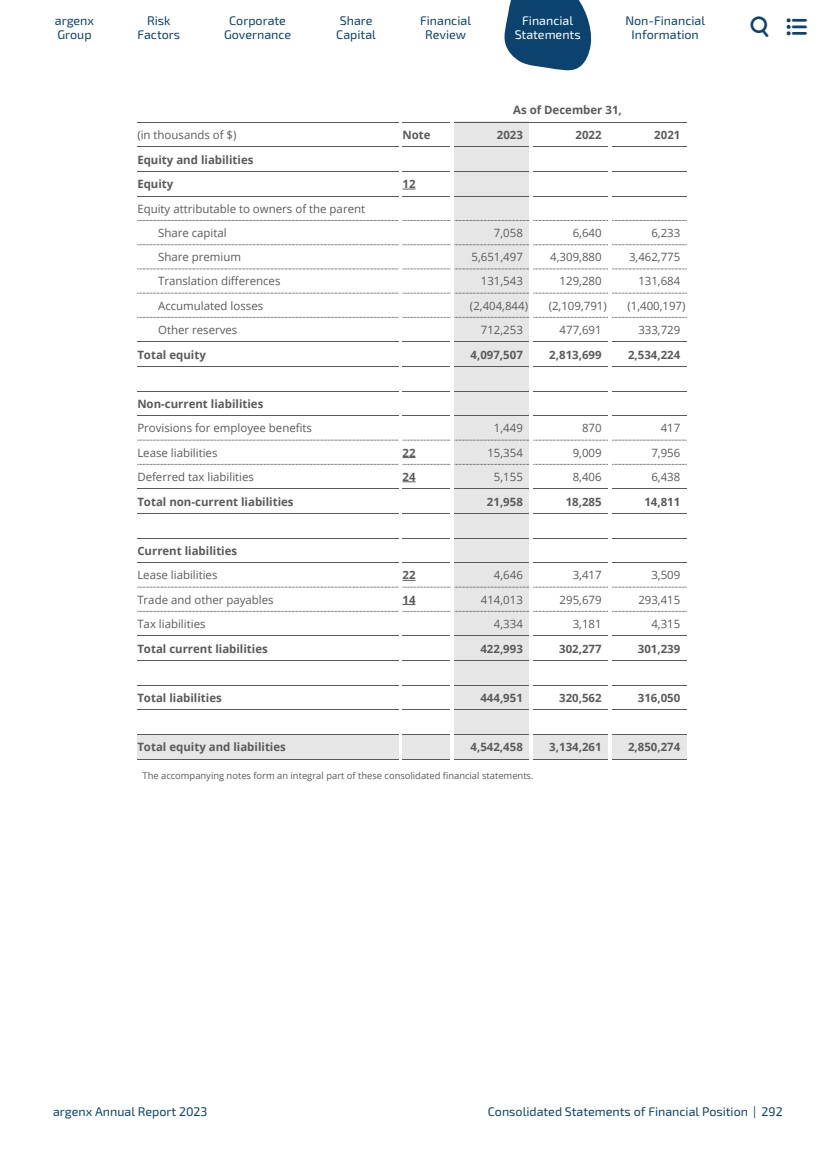
| 自2023年12月31日的資產負債表日期以來,本集團的財務業績或財務狀況沒有發生重大變化。 有關更多信息,請參閲集團的列報和風險 因素一節,以及我們的合併財務報表的注29“承諾”在 “綜合財務報表-截至2023年12月31日的年度”。 競爭地位 我們參與的一個高度創新的行業,其特點是對疾病生物學的瞭解迅速增長,技術迅速變化,強大的知識產權 進入壁壘,以及參與新療法的創造、開發和商業化的多家公司。這些公司中的許多都是高度複雜的 ,並且經常相互進行戰略合作。 自身免疫領域的競爭非常激烈,涉及多種單抗、其他生物製品 以及許多不同公司已經上市或正在開發的小分子,包括大型製藥公司。我們與範圍廣泛的生物製藥公司競爭,這些公司正在開發治療GMG和其他自身免疫性疾病的產品,包括與VYVGART屬於同一類別的產品,以及與我們的一些候選產品相似的產品。我們知道有幾種FcRN 抑制劑正在臨牀開發或上市。競爭激烈的產品發佈可能會 侵蝕我們產品的未來銷售,包括我們現有的產品和目前正在開發的產品,或者導致意外的產品過時。此類產品的發佈仍在繼續,潛在的競爭產品正處於不同的開發階段。隨着競爭對手推出新產品,我們還可能面臨使用有限的國際輸液地點的競爭,特別是在新市場 。我們無法準確預測 推出治療疾病和疾病的競爭產品的時間或影響,這些疾病和情況與我們的產品或候選產品治療的疾病和情況類似。此外,我們的競爭對手還與我們競爭,在招聘和留住合格的科學和管理人員、建立臨牀試驗場地和患者登記方面與我們競爭,以及在獲取與我們的產品開發互補或必要的技術方面與我們競爭。請參閲“我們的藥物發現和開發工作面臨着激烈的競爭”一節。有關我們面臨的競爭的更多 詳細信息。 1.2.3 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年年度報告戰略和目標|32 |
| 我們的產品和候選產品 下表彙總了截至本年度報告發布之日我們的主導產品和候選產品組合的關鍵信息。 自體免疫渠道的廣度和深度 神經血液和風濕病皮膚病腎臟病適應症未披露 計劃適應症臨牀前第一階段概念驗證註冊商業 ARGX-109未披露 ARGX-213未披露 ARGX-220未披露 ARGX-119先天性肌無力綜合徵 Empasiprubart 多灶性運動神經病 延遲性移植物Function Dermatomyositis gMG ITP VYVGART Hytrulo gMG CIDP Efgartigimod Thyroid眼病 大皰性類天皰瘡 肌炎,Asys,DM) 新冠肺炎膜性腎病後POTS ANCA相關性小血管炎1) 抗體介導的排斥反應 狼瘡腎病 VYVGART GMG批准 我們批准的治療GMG的兩種藥物是VYVGART和VYVGART SC。VYVGART是一種FcRN 阻滯劑,在美國、歐盟、以色列、英國、中國大陸中國和加拿大獲批用於治療抗乙酰膽鹼受體抗體陽性(AChR-AB+)的成人GMG,在日本用於治療對類固醇或非類固醇免疫抑制療法(ITS)沒有足夠反應的成人GMG,包括血清陰性患者。我們的第二個產品VYVGART SC是Gefartigimalfa和重組人透明質酸酶PH20(RHuPH20)的皮下組合,Halozyme的Enhanze®SC藥物釋放 技術。它已被批准用於治療患有GMG的成人,這些人在美國是AChR-AB+AS VYVGART HYTRULO,在歐盟和英國是VYVGART SC。它還在日本被批准為VYVDURA,用於治療對類固醇或非類固醇類藥物反應不足的成人GMG患者,包括血清陰性患者。 1.3 1.3.1 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查聲明 財務非財務 信息 argenx 2023年年度報告我們的產品和候選產品|33 |
| gMG是一種罕見的慢性自身免疫性疾病,經常導致衰弱和潛在威脅生命的肌肉無力。gMG的一個關鍵驅動因素是抗乙酰膽鹼受體抗體(AChR)自身抗體在神經肌肉接頭處的作用。VYVGART是一種結合FcRn的人IgG 1抗體片段,通過減少循環IgG抗體發揮作用。 VYVGART的批准基於全球III期ADAPT臨牀試驗的結果, 該試驗發表在2021年7月出版的《柳葉刀神經病學》(來源:Howard JF Jr et al.,efgartigimod在全身性重症肌無力(ADAPT)患者中的安全性、療效和耐受性:一項多中心、隨機、安慰劑對照、III期試驗柳葉刀神經病學。2021;20:526—36)。 ADAPT臨牀試驗表明,與安慰劑相比,使用efgartigimod治療後,AChR—AB + gMG患者的日常生活活動能力(MG—ADL)評分顯著增加(67.7% vs. 29.7%;p |
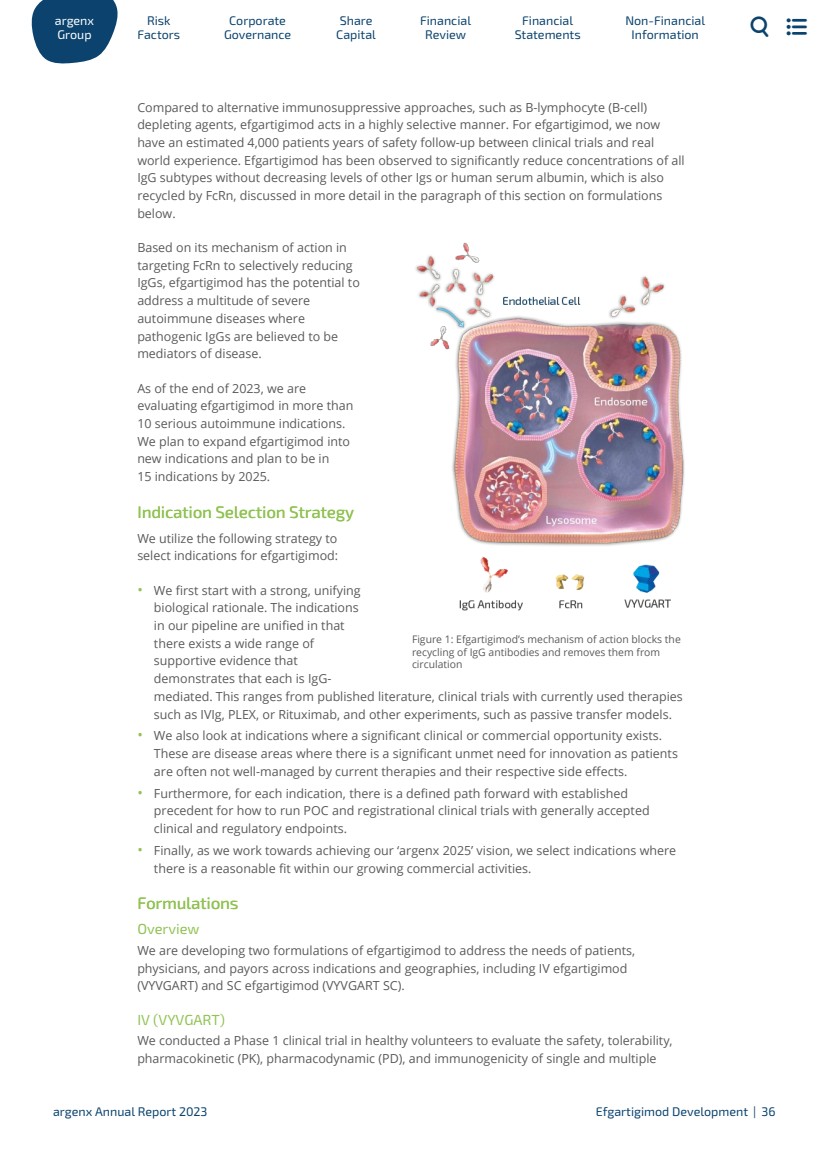
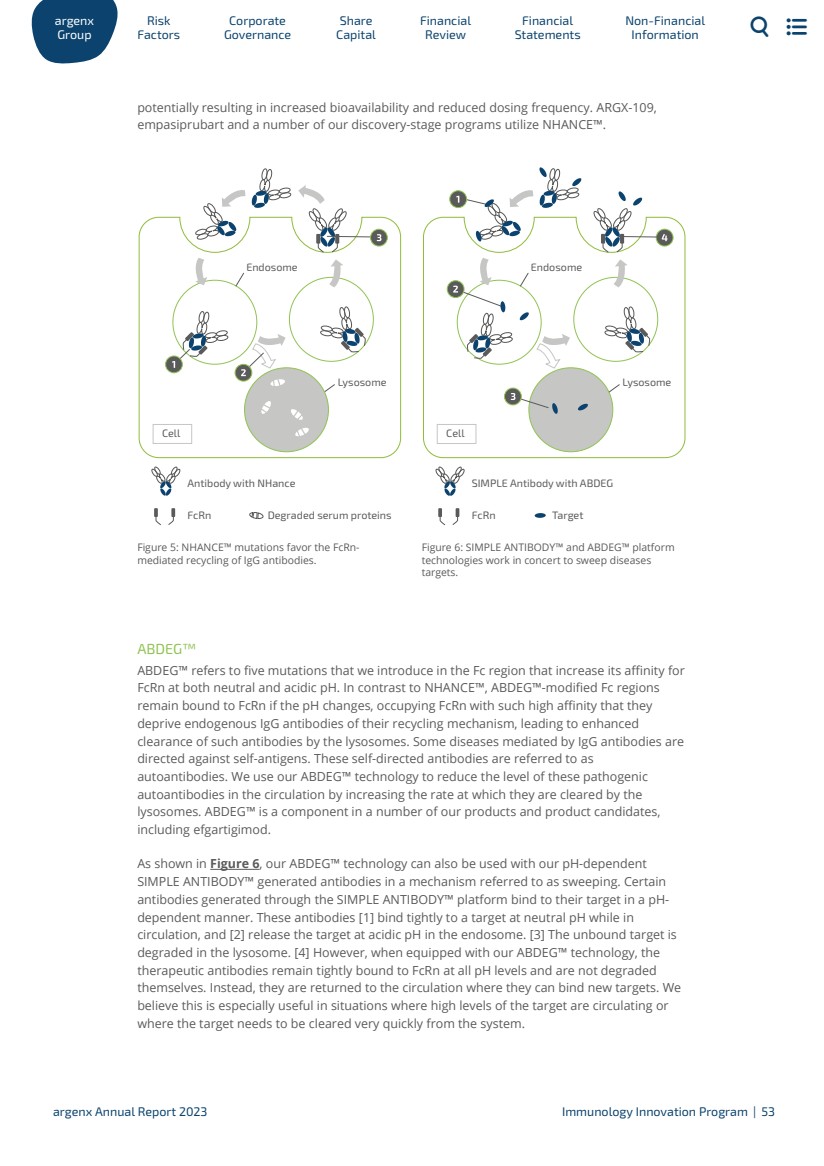
| Development and commercialization may also be done through collaborations with third parties. In January 2021, we entered into an exclusive out-license agreement with Zai Lab (Zai Lab Agreement), a commercial-stage biopharmaceutical company, for the development and commercialization of efgartigimod in Greater China, (which includes Mainland China, Hong Kong, Taiwan and Macau, Greater China). Zai Lab announced approval of VYVGART in Mainland China in June 2023 for the treatment of adult gMG patients. Under the Zai Lab Agreement, we received and continue to be eligible for certain milestone payments and royalties based on annual net sales of efgartigimod in Greater China. In October 2021, we announced an exclusive distribution agreement with Medison to commercialize efgartigimod for gMG in Israel (Medison Agreement). Medison filed for and obtained approval for VYVGART in Israel in April 2023. On June 6, 2022 we announced an exclusive multi-regional agreement with Medison to commercialize efgartigimod in 14 countries, including Poland, Hungary, Slovenia, Czech Republic, Romania, Bulgaria, Lithuania, Croatia, Slovakia, Estonia, Latvia, Greece, and Cyprus, for the treatment of adult patients with gMG (Medison Multi-Regional Agreement). In January 2022, we entered into a partnership agreement with Genpharm Services FZ-LLC (Genpharm), , under which Genpharm shall purchase VYVGART from us for the resale in the Gulf Cooperation Council (GCC) on an exclusive basis for Genpharm’s own account and own name (Genpharm Agreement). In 2023, we entered into the Handok Agreement with Handok for the distribution of VYVGART in South Korea. We intend to sign additional distribution partnerships for other territories. For a discussion of total revenues by geographic market, please see “18. Segment reporting” in our consolidated financial statements. Pre-Approval Access Program We are committed to improving the lives of people suffering from rare diseases. We are driven to discover new treatment approaches in autoimmunity and fueled by the resilience of patients to urgently deliver them. We aim to do this in partnership; we listen to patients, supporters and advocacy communities, and we hear their stories. Their insights guide us as we develop our investigational therapies and motivate us to advance the understanding of rare diseases. We implemented a pre-approval access program (PAA) on February 21, 2021 through which investigational therapies are made available in certain circumstances to treat gMG patients who are unable to participate in an ongoing clinical trial. In 2023, we approved access to the PAA for over 330 gMG patients in 14 countries. The PAA program remains open in countries where VYVGART is not yet launched or reimbursed. Efgartigimod (formerly ARGX-113) Development Mechanism of Action As shown in Figure 1, efgartigimod is a human IgG1 Fc fragment equipped with our ABDEG™ mutations that is designed to target the FcRn and reduce IgG. FcRn is foundational to the immune system and functions to recycle IgG, extending its serum half-life over other Igs that are not recycled by FcRn. IgGs that bind to FcRn are rescued from lysosomal degradation. By binding to FcRn, efgartigimod can reduce IgG recycling and increase IgG degradation. 1.3.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Development | 35 |
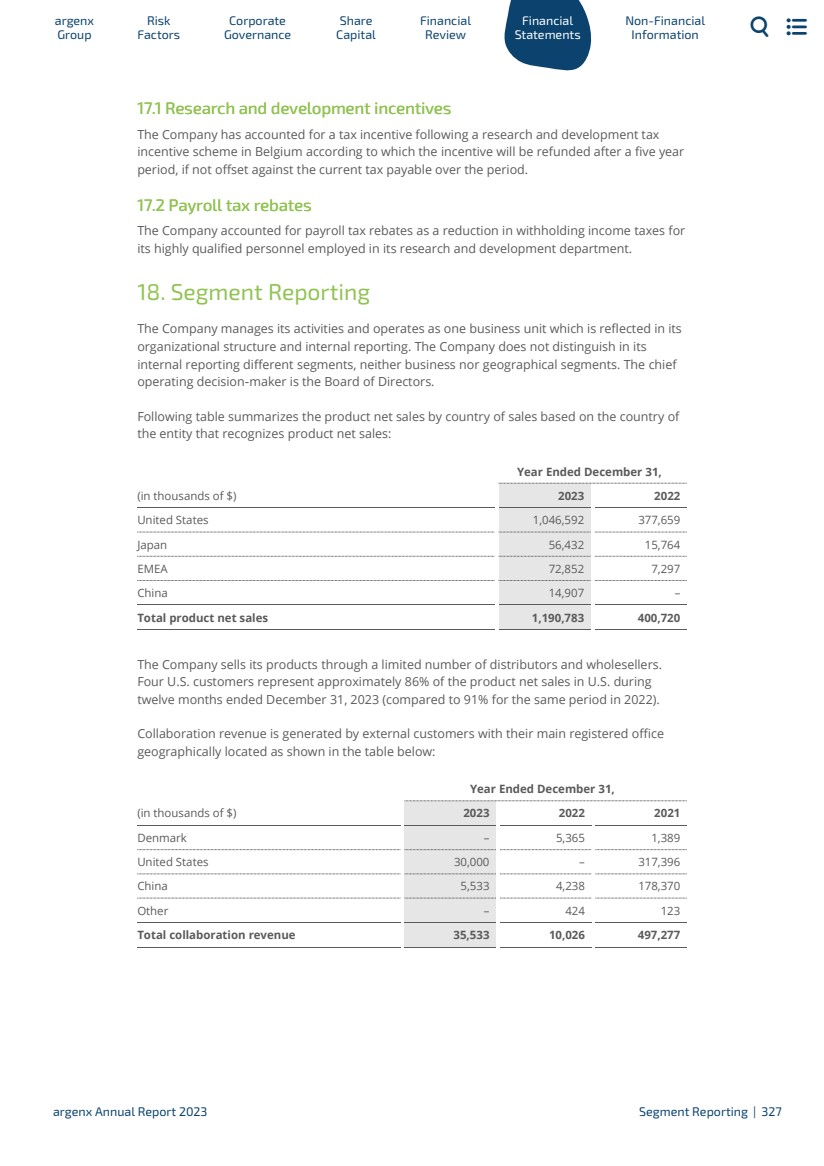
| Figure 1: Efgartigimod’s mechanism of action blocks the recycling of IgG antibodies and removes them from circulation Compared to alternative immunosuppressive approaches, such as B-lymphocyte (B-cell) depleting agents, efgartigimod acts in a highly selective manner. For efgartigimod, we now have an estimated 4,000 patients years of safety follow-up between clinical trials and real world experience. Efgartigimod has been observed to significantly reduce concentrations of all IgG subtypes without decreasing levels of other Igs or human serum albumin, which is also recycled by FcRn, discussed in more detail in the paragraph of this section on formulations below. Based on its mechanism of action in targeting FcRn to selectively reducing IgGs, efgartigimod has the potential to address a multitude of severe autoimmune diseases where pathogenic IgGs are believed to be mediators of disease. As of the end of 2023, we are evaluating efgartigimod in more than 10 serious autoimmune indications. We plan to expand efgartigimod into new indications and plan to be in 15 indications by 2025. Indication Selection Strategy We utilize the following strategy to select indications for efgartigimod: · We first start with a strong, unifying biological rationale. The indications in our pipeline are unified in that there exists a wide range of supportive evidence that demonstrates that each is IgG-mediated. This ranges from published literature, clinical trials with currently used therapies such as IVIg, PLEX, or Rituximab, and other experiments, such as passive transfer models. · We also look at indications where a significant clinical or commercial opportunity exists. These are disease areas where there is a significant unmet need for innovation as patients are often not well-managed by current therapies and their respective side effects. · Furthermore, for each indication, there is a defined path forward with established precedent for how to run POC and registrational clinical trials with generally accepted clinical and regulatory endpoints. · Finally, as we work towards achieving our ‘argenx 2025’ vision, we select indications where there is a reasonable fit within our growing commercial activities. Formulations Overview We are developing two formulations of efgartigimod to address the needs of patients, physicians, and payors across indications and geographies, including IV efgartigimod (VYVGART) and SC efgartigimod (VYVGART SC). IV (VYVGART) We conducted a Phase 1 clinical trial in healthy volunteers to evaluate the safety, tolerability, pharmacokinetic (PK), pharmacodynamic (PD), and immunogenicity of single and multiple ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Development | 36 |
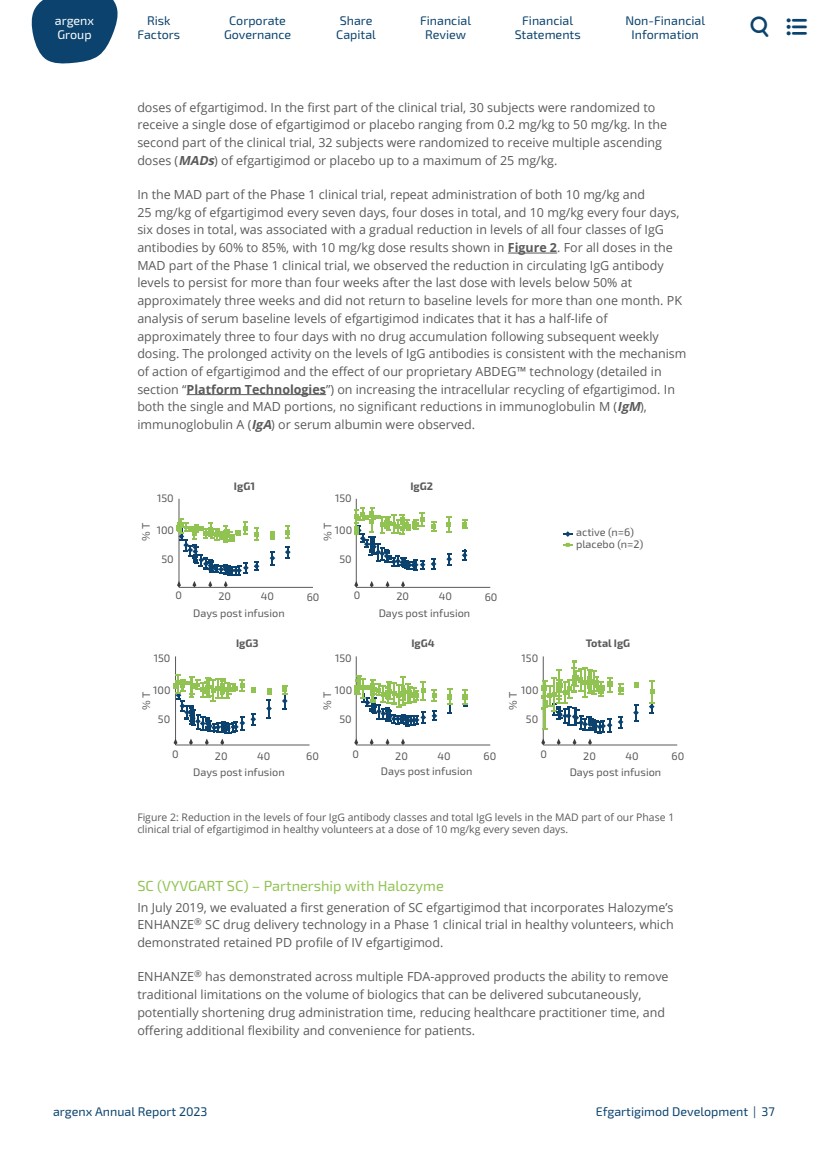
| doses of efgartigimod. In the first part of the clinical trial, 30 subjects were randomized to receive a single dose of efgartigimod or placebo ranging from 0.2 mg/kg to 50 mg/kg. In the second part of the clinical trial, 32 subjects were randomized to receive multiple ascending doses (MADs) of efgartigimod or placebo up to a maximum of 25 mg/kg. In the MAD part of the Phase 1 clinical trial, repeat administration of both 10 mg/kg and 25 mg/kg of efgartigimod every seven days, four doses in total, and 10 mg/kg every four days, six doses in total, was associated with a gradual reduction in levels of all four classes of IgG antibodies by 60% to 85%, with 10 mg/kg dose results shown in Figure 2. For all doses in the MAD part of the Phase 1 clinical trial, we observed the reduction in circulating IgG antibody levels to persist for more than four weeks after the last dose with levels below 50% at approximately three weeks and did not return to baseline levels for more than one month. PK analysis of serum baseline levels of efgartigimod indicates that it has a half-life of approximately three to four days with no drug accumulation following subsequent weekly dosing. The prolonged activity on the levels of IgG antibodies is consistent with the mechanism of action of efgartigimod and the effect of our proprietary ABDEG™ technology (detailed in section “Platform Technologies”) on increasing the intracellular recycling of efgartigimod. In both the single and MAD portions, no significant reductions in immunoglobulin M (IgM), immunoglobulin A (IgA) or serum albumin were observed. IgG1 % T % T IgG2 placebo (n=2) active (n=6) IgG3 % T % T % T IgG4 Days post infusion Days post infusion Days post infusion Days post infusion Days post infusion 0 50 100 150 50 100 150 50 100 150 50 100 150 50 100 150 20 40 60 0 20 40 60 0 20 40 60 0 20 40 60 0 20 40 60 Total IgG Figure 2: Reduction in the levels of four IgG antibody classes and total IgG levels in the MAD part of our Phase 1 clinical trial of efgartigimod in healthy volunteers at a dose of 10 mg/kg every seven days. SC (VYVGART SC) – Partnership with Halozyme In July 2019, we evaluated a first generation of SC efgartigimod that incorporates Halozyme’s ENHANZE® SC drug delivery technology in a Phase 1 clinical trial in healthy volunteers, which demonstrated retained PD profile of IV efgartigimod. ENHANZE® has demonstrated across multiple FDA-approved products the ability to remove traditional limitations on the volume of biologics that can be delivered subcutaneously, potentially shortening drug administration time, reducing healthcare practitioner time, and offering additional flexibility and convenience for patients. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Development | 37 |
| In 2020, we expanded the existing global collaboration and license agreement with Halozyme. Under the expansion, we gained the ability to access Halozyme’s ENHANZE® SC drug delivery technology for three additional exclusive targets upon nomination bringing the total to six potential targets under the collaboration. To date, two targets have been nominated including FcRn and C2. In March 2022, we announced our Phase 3 ADAPT-SC clinical trial evaluating SC efgartigimod achieved the primary endpoint of total IgG reduction from baseline at day 29, demonstrating statistical non-inferiority to VYVGART IV formulation in gMG patients. Based on these results, we received approval of VYVGART SC for the treatment of adult patients with gMG in the U.S., the EU, the UK and Japan. Currently, we are developing a pre-filled syringe presentation for the same SC formulation using the Halozyme technology, to allow for a convenient delivery and the potential for self-administration, reducing the healthcare practitioner time and further increasing flexibility and convenience for patients. As a next step in patient convenience, we have also started the development of a high-volume auto-injector. SC – Partnership with Elektrofi In April 2021, we entered into a collaboration and license agreement with Elektrofi to explore a high concentration technology for efgartigimod and up to one additional target. Please refer to “Our Exclusive License with Elektrofi for efgartigimod” for more information. Efgartigimod Indications gMG Overview gMG is a rare and chronic autoimmune disease where IgG autoantibodies disrupt communication between nerves and muscles, causing debilitating and potentially life-threatening muscle weakness. In myasthenia gravis (MG), IgG autoantibodies either bind and occupy or cross-link and internalize the receptor on the muscle cells, thereby preventing the binding of acetylcholine, the signal sent by the nerve cell. In addition, these autoantibodies can cause destruction of the neuromuscular junction by recruiting complement, a potent cell-destroying mechanism of the human immune system. The muscle weakness associated with MG usually presents initially in ocular muscles and can then spread into a generalized form affecting multiple muscles, known as gMG. Approximately 85% of people with MG progress to gMG within 24 months (source: Behin et al. New Pathways and Therapeutics Targets in Autoimmune Myasthenia Gravis. J Neuromusc Dis 5. 2018. 265-277). MG in the ocular form initially causes droopy eyelids and blurred or double vision due to partial paralysis of eye movements. As MG becomes generalized it affects muscles in the neck and jaw, causing problems in speaking, chewing and swallowing. MG can also cause weakness in skeletal muscles leading to problems in limb function. In the most severe cases, respiratory function can be weakened to the point where it becomes life-threatening. These respiratory crises occur at least once in the lives of approximately 15% to 20% of MG patients. The U.S. prevalence of MG is estimated at approximately 20 cases per 100,000 (source: Philips et al, Ann NY Acad Sci. 2003). Patients with confirmed AchR antibodies account for approximately 85% of the total gMG population (Behin et al. New Pathways and Therapeutics Targets in Autoimmune Myasthenia Gravis. J Neuromusc Dis 5. 2018. 265-277). 1.3.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Indications | 38 |
| In May 2020, we announced positive topline results from the pivotal ADAPT clinical trial of efgartigimod for the treatment of gMG. The topline results from the ADAPT clinical trial showed that efgartigimod was well-tolerated, demonstrated clinically meaningful improvements in strength and quality of life measures, and provided the option of an individualized dosing schedule for gMG patients. The full Phase 3 ADAPT results were published in The Lancet Neurology in July 2021. The data from the ADAPT clinical trial and the subsequent open-label extension (OLE) (ADAPT+) formed the basis for the regulatory approvals of VYVGART in the U.S., Japan, the EU, Mainland China, Israel, the UK and Canada. On March 22, 2022, we announced positive topline results from the Phase 3 ADAPT-SC s clinical trial, a registrational non-inferiority bridging clinical trial of SC efgartigimod for the treatment of gMG. SC efgartigimod achieved the primary endpoint of total IgG reduction from baseline at day 29, demonstrating statistical noninferiority to VYVGART IV formulation in gMG patients. Based on these results, we received regulatory approval in the U.S. in June 2023, in the EU in September 2023, in Japan in January 2024 and in the UK in February 2024. Other clinical trials We are currently evaluating alternative dosing regimens of IV efgartigimod in adult gMG patients in the ADAPT NXT clinical trial. In addition, a clinical trial of IV efgartigimod in pediatric gMG patients is ongoing. In 2022, a Phase 1 clinical trial evaluating the effect of efgartigimod or placebo on immune response to the polyvalent pneumococcal vaccine (PNEUMOVAX 23) was completed. In 2024, we plan to initiate registrational clinical trials to expand VYVGART label into broader MG populations, including in seronegative patients. CIDP Overview CIDP is a chronic autoimmune disorder of peripheral nerves and nerve roots caused by an autoimmune-mediated destruction of the myelin sheath, or myelin producing cells, insulating the axon of the nerves and enabling speed of signal transduction. The cause of CIDP is unknown, but abnormalities in both cellular and humoral immunity have been shown. CIDP is a chronic and progressive disease: onset and progression occur over at least eight weeks in contrast with the more acute Guillain-Barré-syndrome. Demyelination and axonal damage in CIDP lead to loss of sensory and/or motor neuron function, which can lead to weakness, sensory loss, imbalance and/or pain. CIDP affects approximately 24,000 patients in the U.S. Most CIDP patients require treatment, the majority currently with IVIg. Glucocorticoids and plasma exchange are used to a lesser extent as they are either limited by side effects upon chronic use, in the case of glucocorticoids, or invasiveness of the procedure and access, which is restricted to specialized centers in case of plasma exchange. Alternative immunosuppressant agents are typically reserved for patients ineligible for or refractory to IVIg, glucocorticoids or plasma exchange. In July 2023, we announced positive topline results from the ADHERE clinical trial evaluating VYVGART SC (efgartigimod alfa and hyaluronidase-qvfc) in adults with CIDP. The clinical trial met its primary endpoint (p=0.000039), demonstrating a significantly lower risk of relapse with VYVGART SC compared to placebo (HR: 0.39 95% CI: 0.25; 0.61). 67% of patients in open-label Stage A demonstrated evidence of clinical improvement (ECI), indicating that IgG autoantibodies play a significant role in the underlying biology of CIDP. VYVGART SC was well-tolerated with a safety profile that is consistent with prior clinical trials and the known profile of VYVGART. The most frequent treatment-related adverse event was ISRs, which occurred in a lower percentage of patients than previous VYVGART SC trials (20% in Stage A; 10% in Stage B). All ISRs were mild to moderate and resolved over time. 99% (226/ ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Indications | 39 |
| 249)合格患者繼續參加ADHERE—Plus OLE臨牀試驗。 ADHERE的詳細數據預計將在即將舉行的醫學會議上公佈。 2023年12月,我們向FDA提交了一份sBLA,用於CIDP的SC efgartigimod,附帶 優先審查憑證。FDA接受sBLA進行優先審查,PDUFA目標日期為2024年6月21日。 原發性ITP 概述 原發性ITP是一種獲得性自身免疫性出血疾病,其特徵是血小板計數低 ( |
| count response in chronic ITP patients. Secondary endpoints were also not met, including additional endpoints on IWG responder status and mean platelet count change from baseline. VYVGART SC was well-tolerated in ADVANCE-SC; the observed safety and tolerability profile was consistent with ADVANCE-IV and the confirmed safety profile of VYVGART and VYVGART SC. Pemphigus Overview PV is an autoimmune disorder associated with mucosal and skin blisters that lead to pain, difficulty swallowing and skin infection. This chronic, potentially life-threatening disease is triggered by IgG autoantibodies targeting desmoglein-1 and -3, which are present on the surface of keratinocytes and important for cell-to-cell adhesion in the epithelium. Autoantibodies targeting desmogleins result in loss of cell adhesion, the primary cause of blister formation in PV. Similar to MG and ITP, disease severity of pemphigus correlates to the amount of pathogenic IgGs targeting desmogleins. Currently, there are an estimated 19,000 pemphigus patients in the U.S., of which an estimated 13,100 patients are suffering from PV. Several disease activity measurements exist for the clinical evaluation of PV patients, including the pemphigus disease area index (PDAI), autoimmune bullous skin disorder intensity score, and the PV activity score (PVAS). The PDAI is reported to have the highest validity and is recommended for use in clinical trials of PV. Phase 3 ADDRESS Clinical Trial In 2020, the registrational ADDRESS clinical trial was initiated of SC efgartigimod for the treatment of PV and PF. This was a randomized, double-blinded, placebo-controlled clinical trial, where the objective was to assess efficacy, safety and tolerability in newly diagnosed or relapsing patients with moderate to severe pemphigus (total of 222 enrolled). Patients were randomized to receive either SC efgartigimod or placebo for 30 weeks. Patients started on concomitant steroids based on what we determined to be the optimized dosing regimen from the Phase 2 POC clinical trial. The primary endpoint assessed the proportion of patients who achieve sustained complete remission on a minimal steroid dose within 30 weeks. The ADDRESS clinical trial evaluated efficacy and safety, including the potential to drive fast onset of disease control and complete remission and the ability to taper corticosteroids. Topline data from the Phase 3 ADDRESS clinical trial were announced in December 2023, in which the results show the proportion of PV patients achieving the primary endpoint of complete remission on CRmin was not significantly different between SC efgartigimod and placebo. We will not pursue additional development in pemphigus and we will prioritize clinical development of efgartigimod in its ongoing severe autoimmune indications. BP Overview BP is the most common autoimmune blistering disease and is driven by autoantibodies affecting the skin. The disease typically affects elderly people and early key symptoms are itch and rash and patients develop fluid-filled blisters during disease progression. The prevalence of BP is 12 per 100,000 adults and the incidence increases with age. BP is associated with a high disease burden and can have a significant impact on the quality of life of patients. The mortality of BP in the U.S. is 2.4% or higher than the mortality in the general population of the same age. There are currently no approved therapies available for BP. First line treatment consists of topical or systemic corticosteroids, which result in substantial morbidity and increased mortality, conventional immunosuppressants as corticosteroid-sparing agents, rituximab and IVIg. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Indications | 41 |
| BP is a well characterized autoimmune disease in which the binding of autoantibodies to hemidesmosomal proteins, BP180 and BP230, initiates a cascade of inflammatory events resulting in blister formation. BP180 and BP230 are involved in the stable attachment of keratinocyte to the underlying matrix. The autoantibody actions include mechanical disruption of keratinocyte adhesion and cytokine release. Immune complex formation initiates complement activation leading to the recruitment mast cells, neutrophils, eosinophils and other immune cells and to the release of proteases and inflammatory mediators. All these effects, which start with the binding of the autoantibodies, induce the blistering observed in BP. BALLAD Clinical Trial We initiated the Phase 2/3 BALLAD registrational clinical trial evaluating SC efgartigimod in BP in 2022. The clinical trial population are newly diagnosed and relapsing patients within one year from diagnosis. Patients are randomized 1-to-1 to receive efgartigimod or placebo for a total duration of 36 weeks. The primary endpoint is the proportion of participants in complete remission while off oral corticosteroids for at least eight weeks at week 36. Secondary endpoints relate to cumulative steroid doses, IGA BP score, time to achieving control of disease activity, change from baseline in average itch, and quality of life measures. In light of ADDRESS results and the comparable biology between PV and BP, we decided to stop enrollment of BALLAD. We will integrate key learnings from ADDRESS and data from already-enrolled patients in BALLAD and we plan to communicate on a revised development plan before end 2024. Myositis Overview Myositis are a rare group of autoimmune diseases that can be muscle specific or affect multiple organs including the skin, joints, lung, gastrointestinal tract and heart. Myositis can be very severe and disabling and have a material impact on quality of life. Initially these Myositis were classified as either DM or polymyositis, but as the underlying pathophysiology of Myositis has become better understood, including through the identification of characteristic autoantibodies, new polymyositis subgroups have emerged. Two of these subtypes are IMNM and ASyS. Proximal muscle weakness is a unifying feature of each Myositis subset. IMNM is characterized by skeletal muscle weakness due to muscle cell necrosis. The muscle weakness is typically symmetrical – on both sides of the body – and affects proximal muscles including hips, thighs, upper arms, shoulder and neck. The muscle weakness can be severe and lead to difficulty in completing daily tasks. Characteristic autoantibodies of IMNM, include anti-signal recognition particle and anti-3-hydroxy-3-methylglutaryl-coenzyme A reductase autoantibodies. ASyS is characterized by muscle inflammation, inflammatory arthritis, interstitial lung disease, thickening and cracking of the hands (“mechanic’s hands”) and Raynaud phenomenon. Autoantibodies associated with ASyS attack tRNA synthetase enzymes and include anti-Jo-1 and anti-PL1 and PL-12 most commonly. DM is characterized by muscle inflammation and degeneration and skin abnormalities, including heliotrope rash, Gottron papules, erythematous, calcinosis and edema. DM is associated with Myositis-specific autoantibodies, including anti-Mi-2, anti-MDA-5, anti-TIF-1γ and others. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Efgartigimod Indications | 42 |
| 目前還沒有FDA批准的治療IMNM或ASYS的方法。IVIG(奧塔康10%)於2021年7月被FDA批准用於治療糖尿病。肌炎患者最常使用大劑量類固醇治療。 ALKIVIA臨牀試驗我們於2022年啟動了SC efgartigimod治療肌炎的註冊ALKIVIA臨牀試驗。這項臨牀試驗計劃招募大約240名患者,分為三種肌炎亞型:IMNM、ASYS和DM。臨牀試驗將分兩個階段進行,對臨牀試驗的第二階段進行分析,包括每個亞型的30名患者,然後只有在臨牀試驗的第二階段觀察到信號時,才進行臨牀試驗的第三階段。 主要終點是治療結束時的總改善評分(TIS)。 關鍵次要終點包括治療結束時的應答率、有效時間、 和TIS中的反應持續時間。其他次要終點包括生活質量和其他功能評分。預計將在2024年下半年對每個亞組中的前30名患者進行中期分析。TED是一種與Graves病和其他自身免疫性甲狀腺疾病相關的自身免疫性眼眶疾病,如橋本氏甲狀腺炎。TED的特點是眼外肌增大、眼眶脂肪組織擴張和眼眶炎症,嚴重時可導致眼球突出、複視或視力喪失。持續的眼眶症狀通常會損害患者長期的生存質量。 大量非臨牀和臨牀證據支持促甲狀腺激素受體(TSHR) 自身抗體在TED的病理中起作用。臨牀證據支持清除自身抗體作為治療TED的一種機制。通過降低免疫球蛋白γ,包括與TED相關的致病免疫球蛋白自身抗體,Egartigimod有望緩解疾病症狀。此外,降低免疫球蛋白可以解決潛在的甲狀腺功能亢進症。當前療法的副作用和耐受性問題,包括類固醇和替普羅單抗(僅FDA批准的生物療法),是許多患者的治療限制 基於合併症和對安全和方便的療法的重大需求仍未得到滿足。 預計將於2024年開始評估efgartigimod在TED中的註冊臨牀試驗。SJD是一種慢性進行性自身免疫性疾病,特徵是淋巴細胞滲透和外分泌腺進行性破壞。B細胞在疾病的發展中起着關鍵作用,這導致了免疫球蛋白自身抗體的產生,特別是針對SSA/Ro,SSB/La核糖核覆合體的抗體。除了眼睛乾燥、口腔乾燥、慢性疼痛和疲勞等症狀外,相當一部分患者還患有腺外系統疾病。目前沒有FDA批准的用於治療SjD. ar Gr g oup enx Factors Risk Go Corporate vernance資本的治療方法 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告Efgartigimod適應症|43 |
| Rho第二階段臨牀試驗(與IQVIA合作) 2023年,我們啟動了第二階段POC臨牀試驗,評估IV efgartigimod治療SJD的效果。Rho臨牀試驗是一項評估IV efgartigimod的隨機、安慰劑對照、雙盲臨牀試驗。這項臨牀試驗招募了大約30名至少患有中度系統性疾病(ESSDAI≥5)的患者。患者必須接受穩定的背景治療,且抗SSA/Ro陽性。在24周的治療期結束時,完成臨牀試驗的參與者可以進入OLE。主要終點是第24周SJD(CRESS;第3項(共 個項目,共 個項目)中的≥3項的迴應)的應答者與相關終結點組合的比例。關鍵的次要終點包括臨牀ESSDAI (臨牀ESSDAI)、Eular Sjögrens綜合徵疾病活動指數(ESSDAI)和Eular Sjögrens患者報告指數(ESSPRI)評分較基線的變化。 Rho臨牀試驗結果預計將於2024年上半年公佈。 新冠肺炎後概述 在先前健康的患者感染SARS-CoV後,出現了POTS後新冠肺炎。POTS後新冠肺炎是一種自主神經系統紊亂, 特徵是站立時心率加快,以及額外的 呼吸急促、頭痛、疲勞、注意力不集中、虛弱和焦慮 症狀。絕大多數患者是15歲至50歲的女性。新冠肺炎後POTS與激活針對自主G蛋白偶聯受體的自身抗體有很強的相關性,這些受體包括β1和β2腎上腺素能受體以及M2和M3 M受體。目前還沒有FDA批准的針對血容量、腎臟鈉水平、心率減慢和血管收縮的對症治療 。 POC Alpha第二階段臨牀試驗(與IQVIA合作) 2022年,我們啟動了安慰劑對照的POC Alpha第二階段臨牀試驗,每週靜脈注射efgartigimod治療由新冠肺炎引發的新POC。共同的主要終點是COMPASS-31和24周治療結束時的Malmöpots症狀評分 。關鍵的次要終點包括Promis疲勞和認知功能較基線的變化,以及患者對變化和嚴重程度的總體印象。其他次要終點包括定量自主神經測試和其他功能評分。 第二階段POC Alpha臨牀試驗結果預計將於2024年上半年公佈。 LN 概述 LN是一種腎臟炎症性自身免疫性疾病,是自身免疫性疾病系統性紅斑狼瘡(SLE)最嚴重和最常見的器官表現之一。在SLE患者中,大約25%到50%的患者在SLE發病時有腎臟疾病的跡象或症狀。大約40%到60%的SLE患者在病程中會發生腎臟損害,具有相當高的發病率或死亡率。致病性自身抗體和補體沉積在SLE,尤其是LN的發病機制中起關鍵作用,腎臟免疫複合體的沉積是該疾病的一個標誌。與LN相關的自身抗體包括抗dsDNA抗體、抗C1q抗體、抗心磷脂抗體、抗Smith抗體和抗核抗體。10-30%的LN患者進展為終末期腎病。口服皮質類固醇和廣泛的免疫抑制劑是目前的護理標準,但並不是一致有效的。Belimumab(苯利司塔)和voclosporin(Lupkynis)被食品和藥物管理局批准用於治療LN. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 argenx 2023年年度報告|44 |
| POC第二階段臨牀試驗(與再鼎醫藥合作) 2023年,我們啟動了一項POC臨牀試驗,以評估靜脈注射Efgartigimod對中國活動期LN患者的療效和安全性。這項臨牀試驗計劃招募大約60名LN III或IV級(有或不有V類)的患者。 主要終點是從基線到治療期結束時尿蛋白肌酐比率(UPCR)的變化。關鍵的次要終點包括患者在療程結束時達到完全(CRR)和部分腎反應(PRR)的比例,以及達到CRR和PRR的時間。其他次要終點包括額外的療效測量、PK、PD、免疫原性、生物標誌物、安全性和生活質量評估。MN概述MN是一種自身免疫性腎小球疾病,是成人腎病綜合徵的最常見原因之一。MN的特徵是免疫複合物沉積引起的腎小球基底膜增厚。多達75%的MN患者具有抗PLA2R的自身抗體。數據高度提示抗PLA2R抗體與MN發病之間存在因果關係。到目前為止已確定的其他靶抗原包括血栓反應蛋白1型結構域包含7A(THSD7A)、神經表皮生長因子樣蛋白-1(NELL-1)和信號素-3B(Sema3B)。20-30%的MN患者進展為終末期腎臟疾病。所有MN患者都得到了最佳的支持性治療,疾病進展的高風險患者還會接受廣泛的免疫抑制藥物治療。目前還沒有批准的治療MN的方法。 POC第二階段臨牀試驗(與再鼎醫藥合作) 2023年,我們啟動了一項POC臨牀試驗,以評估靜脈注射efgartigimod對中國原發MN(PMN)患者的療效和安全性。這項臨牀試驗計劃招募最多72名PMN患者。臨牀試驗將包括兩個階段:主要臨牀試驗的雙盲期(DB) ,隨後是可選的OLE期。主要終點是在抗PLA2R抗體血清陽性人羣中,從基線到治療期末UPCR的變化。關鍵的次要終點包括總體人羣的UPCR從基線到治療期末的變化,總體人羣和抗PLA2R抗體陽性人羣中在治療期結束時達到完全緩解和部分緩解的參與者的比例,以及總體人羣和抗PLA2R抗體陽性人羣中完成緩解和部分緩解的時間。其他次要終點包括額外的療效測量、PK、PD、免疫原性、生物標記物、安全性和生活質量評估。 其他Efgartigimod適應症 AMR AMR是一種影響移植器官的自身免疫性疾病,可能導致 移植物丟失。同種異體腎移植中的AMR是由供者特異性抗體(DSA)驅動的,供者特異性抗體通常針對同種異體內皮細胞表達的HLA抗原。DSA可以通過不同的機制誘導微血管炎症,這是AMR的組織病理學特徵。微血管炎症導致器官功能喪失,如果持續下去,可能導致同種異體移植物丟失。對有效治療的未得到滿足的需求非常高,AMR 是腎移植失敗的主要原因。目前還沒有批准的治療AMR. ar Gr g oup enx Factors Risk Go Corporate vernance資本的方法 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告Efgartigimod適應症|45 |
| AAV-與IQVIA合作 ANCA相關性血管炎(AAV)是一種自身免疫性疾病,其特徵是體內小血管的炎症和損傷。AAV有三種不同的亞型:肉芽腫合併多血管炎、顯微鏡下的多血管炎和嗜酸性肉芽腫合併多血管炎(EGPA)。多血管炎或顯微鏡下的多血管炎通常分別與PR3或MPO自身抗體的存在有關。這些自身抗體在疾病中起着關鍵作用,它們與中性粒細胞的結合啟動了一系列炎症過程。患者經常出現乏力、肌肉疼痛、發熱、腹痛和尿血等症狀,但許多患者會發展成腎、肺或心血管系統嚴重受損的危及器官或生命的疾病。 FDA批准了多種治療方法,在糖皮質激素的基礎上,美妥昔單抗被認為是誘導和維持AAV的主要治療方法。 再鼎醫藥 根據再鼎醫藥協議,再鼎醫藥獲得了efgartigimod在大中國的獨家開發權和商業化。再鼎醫藥還將為我們的全球Efgartigimod三期臨牀試驗貢獻患者。我們與再鼎醫藥的戰略合作使我們能夠 加快將Egartigimod開發為新的自身免疫適應症,再鼎醫藥 擔任第二階段POC臨牀試驗的運營負責人。 2022年,再鼎醫藥在MN和LN啟動了第二階段POC臨牀試驗,這兩個臨牀試驗都屬於 新興的腎臟病適應症。這是在完成了第一階段PK/PD臨牀試驗以支持GMG在大陸批准用於中國的efgartigimod,以及 獲得監管部門批准將中國患者納入我們的全球第三階段臨牀試驗後完成的。 我們與再鼎醫藥合作,繼續評估根據再鼎醫藥協議在大中國啟動的更多POC臨牀試驗,以加快 efgartigimod的全球開發。 IQVIA 2021年12月2日,我們與IQVIA簽訂了戰略資產開發協議(資產 開發協議)。根據資產開發協議,IQVIA 將通過 高級外包模式為efgartigimod提供資產和指標開發服務。此類服務包括但不限於:整體產品 適應症開發戰略、臨牀試驗方案設計、我們選擇的efgartigimod適應症的臨牀開發計劃的設置、執行和監督。 為了支持和鼓勵IQVIA快速和創新地提供服務,資產 開發協議包含基於IQVIA的 業績的創新回報和獎金計劃。新冠肺炎之後的POTS和aav是我們在資產開發Agreement. ar Gr g oup enx Factors Risk Go Corporate vernance資本項下確定的進一步發展的指標 共享財務 審查報表 財務非財務 信息 argenx 2023年年報|46 |
| Clinical Trial Stage Indication Patients Primary Endpoint Status ADAPT ADDRESS BALLAD ALKIVIA RHO ALPHA In partnership with Zai Lab In partnership with Zai Lab Clinicaltrial to start in 2024 Other clinicaltrials ADVANCE-SC ADVANCE-IV Registrational Registrational Registrational Registrational Registrational Registrational Registrational Registrational Registrational PoC PoC PoC PoC PoC PoC gMG gMG CIDP ITP ITP PV and PF BP Myositis TED Primary SjD POTS post-COVID19 LN MN The proportion of responders based on the Myasthenia Gravis Activities of Daily Living (MG-ADL) score The proportion of responders based on the Myasthenia Gravis Activities of Daily Living (MG-ADL) score The hazard ratio for the time to first adjusted INCAT deterioration The proportion of patients that achieved sustained platelet response The proportion of patients that achieved sustained platelet response The proportion of patients who achieve complete remission on a minimal steroid dose at 30 weeks The proportion of participants in complete remission while off oral corticosteroids for at least eight weeks at week 36 The total improvement score (TIS) at the end oftreatment period The proportion of responders to the Composite of Relevant Endpoints for SjD (CRESS; response on ≥3 out of 5 items) at week 24 The co-primary endpoints are 1) COMPASS-31 and 2) the Malmö POTS Symptom score at the end ofthe 24-week treatment period The change in urine protein creatinine ratio (UPCR) from baseline to end of the treatment period The change in urine protein creatinine ratio (UPCR) from baseline to end of the treatment period in the anti-PLA2R Ab seropositive population Marketed Marketed sBLA accepted by FDA Positive Topline Data Did not meet primary endpoint, analysis ongoing Did not meet primary endpoint, evaluation of efgart in PV and PF stopped. Analysis ongoing for path forward Ongoing Study results expected first half 2024 Ongoing Study results expected first half 2024 Ongoing Study results expected in 2025 Ongoing Study results expected in 2025 IND submission planned for 2Q 2024 322 131 222 Appr. 240 Appr. 30 53 Appr. 60 Appr. 70 AMR AAV 1) ADAPT-SC ADHERE Ongoing Interim analysis expected second half 2024 Empasiprubart (formerly ARGX-117) Development Mechanism of Action Empasiprubart is a highly differentiated therapeutic monoclonal antibody (mAb) targeting C2 equipped with our proprietary NHANCE™ mutations. By addressing a novel target at the intersection of the complement and lectin pathways of the complement cascade, we believe empasiprubart represents a broad pipeline opportunity across several severe autoimmune indications. Activation of the classical and lectin pathway of complement may contribute to tissue damage and organ dysfunction in a number of autoimmune inflammatory diseases and ischemia-reperfusion conditions. Targeting C2 also leaves the alternative pathway of the complement system intact, which is an important component of the innate defense system. Empasiprubart exhibits both pH- and calcium dependent binding. These unique characteristics enable empasiprubart to capture free C2 in circulation and release it in the endosome to be sorted for degradation in the lysosome. Empasiprubart is equipped with NHANCE™ mutations increasing its affinity for FcRn and allowing it to recycle back into circulation to capture more C2. 1.3.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Empasiprubart Development | 47 |
| 作為IIP的一部分,我們獲得了Empasiprubart的權利。Argenx和Broteio在2017年啟動了一項 合作,在烏得勒支大學的支持下進行研究, 演示empasiprubart機制的臨牀前POC。基於根據此合作協議生成的有希望的臨牀前數據,我們行使了獨家的 選項來許可該計劃,並承擔了進一步開發和 商業化的責任。 除了靜脈注射配方外,我們獨家獲得了Halozyme的Enhanze®SC藥物用於C2靶標的遞送技術。 細胞 ARGX-117 pH 7.4鈣:1.5 mm 溶酶體 FcRN C2 pH和Ca~(2+)開關 最佳循環 無效應 功能 圖3: 左:Empasiprubart顯示pH和鈣依賴的靶標結合。 右:Empasiprubart配備了NHANCE™突變,增加了其在酸性和pH條件下對FcRN的親和力 允許其循環再循環。 Empasiprubart適應症 MMN 概述 MMN是一種衰弱的神經肌肉自身免疫性疾病,其特徵是由於運動神經元退化而緩慢 進行性肌肉無力。它主要影響手部和前臂,主要發生在男性,確診年齡中位數約為40歲。診斷需要大約一年半的時間,經常被誤診為肌萎縮側索硬化症。據估計,美國約有13,000名MMN患者,這一數字還在增加。MMN的特殊病理生理特徵包括存在針對神經節苷脂GM1的IgM自身抗體 和傳導阻滯,即作用電位沿軸突的傳播障礙。GM1在神經系統中廣泛表達,尤其是在Ranvier結節周圍的神經元和雪旺細胞。靜脈注射免疫球蛋白是唯一被批准的治療MMN的藥物,需要經常服用以應對疾病的進展性。 第一階段數據 我們進行了IV和SC Empasiprubart的第一階段健康志願者臨牀試驗。這項首個人類臨牀試驗是一項雙盲安慰劑對照臨牀試驗,旨在評估大劑量恩派普魯巴特在102 healthy ar Gr g oup enx Factors Risk Go Corporate vernance Capital中的安全性、耐受性、PK和PD。 Share Financial 回顧報表 財務非財務 信息 Argenx 2023年年度報告Empasiprubart Development|48 |
| 研究對象。在單次遞增劑量部分,我們評估了70名受試者,並測試了靜脈注射高達80 mg/kg和SC高達60 mg/kg的劑量。在臨牀試驗的MAD部分,我們評估了32名受試者,以瞭解重複給藥的安全性和耐受性 ,特別是生成了一個數據集,以最佳地為PK/PD模型提供信息。 單次和多次給藥恩巴普魯巴特或安慰劑都具有良好的安全性和耐受性,支持患者臨牀試驗中臨牀試驗藥物的研究。 我們觀察到遊離C2水平隨劑量的下降而下降。在一次劑量30 mg/kg恩派西普魯巴特後,遊離C2水平在100天以上下降了95%。在臨牀試驗的MAD部分 ,我們可以達到完全補體阻斷,同時將遊離C2水平降低99%以上。 在分析了第一階段的數據,以及觀察到的良好的安全性和耐受性概況 以及一致的PK/PD概況後,我們於2021年在MMN啟動了POC第二階段臨牀試驗。 中期結果第二階段POC ARDA臨牀試驗 2023年6月,argenx宣佈計劃與MMN的Empasiprubart第二階段ARDA臨牀試驗一起推進到第二隊列。這一決定是在一次獨立數據監測委員會(IDMC)會議對第一劑量隊列進行計劃的中期分析之後做出的。 IDMC審查了參加ARDA臨牀試驗第一組隊列的所有患者(n=22)的中期安全性數據,其中包括完成完整16周治療的9名患者 。IDMC確認恩派普魯巴特的安全性和耐受性良好,與第一階段臨牀試驗的結果一致,並建議進入第二隊列。結合觀察到的早期療效信號,支持安派西普魯巴特在MMN中的POC,Argenx啟動了ARDA臨牀試驗的第二個隊列。 2024年1月,Argenx宣佈了在MMN中建立POC的第二階段POC Arda臨牀試驗的第一個隊列(n=22)的陽性數據。Empasiprubart顯示,與安慰劑相比,靜脈注射免疫球蛋白救援的需求減少了91%[HR:0.09 95% CI(0.02;0.044)]. In total, the ARDA clinical trial is expected to enroll 48 patients across two cohorts. The clinical trial's objective, in addition to assessing safety and efficacy of empasiprubart, is to populate a PK/PD model to inform the Phase 3 clinical trial dose selection. Phase 2 ARDA Clinical Trial Design The Phase 2 POC ARDA clinical trial is a randomized, double-blinded, placebo-controlled multicenter clinical trial to evaluate the safety and tolerability, efficacy, PK, PD, and immunogenicity of two dose regimens of empasiprubart in adults with MMN. The clinical trial consists of an IVIg dependency and monitoring period and two 16-week treatment cohorts of 24 MMN patients receiving empasiprubart or placebo in a 2x1 randomization. The dosing for Cohort 2 was established after a planned interim analysis of the first nine patients to complete the 16-week treatment period from Cohort 1. The primary endpoint is safety and tolerability. Additional endpoints include time to IVIg retreatment, biomarker analyses of C2 levels, and changes in measurements on key functional scores (modified medical research council (mMRC)-10 sum score, grip strength, MMN-RODS) as well as several patient-reported quality of life outcome measures (fatigue severity score (FSS), chronic acquired polyneuropathy patient-reported index (CAP-PRI), and values of the patient global impression change (PGIC) scale). DGF Overview DGF, a complication after kidney transplantation, is defined as the need for dialysis in the first week after transplant. DGF occurs in up to 40% of patients receiving a deceased donor graft, and is associated with worse long-term transplant outcomes. DGF is often the clinical ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Empasiprubart Development | 49 |
| representation of ischemia reperfusion injury, in which the classical and lectin complement pathways play an important role, as shown by compelling evidence from both (in-house) in vitro and in vivo preclinical, and clinical trials. There are currently no approved therapies to reduce DGF risk. Furthermore, there is a well-established process to measure kidney function and DGF, and to establish POC and achieve registration. On this basis, combined with the significant unmet medical need, we have chosen DGF after kidney transplantation as second indication for empasiprubart. Phase 2 POC VARVARA Clinical Trial The Phase 2 POC VARVARA clinical trial was initiated in 2023 and is a randomized, placebo-controlled, double-blinded clinical trial to evaluate the efficacy, safety and tolerability of empasiprubart in improving allograft function in recipients at risk for DGF. The clinical trial will include approximately 102 recipients of an at-risk deceased donor kidney. After a short screening period of |
| ARGX-119 is the first and highly specific agonist mAb targeting human MuSK being developed for patients with neuromuscular disease, such as CMS and ALS. This mAb is derived from llamas and discovered using the argenx SIMPLE ANTIBODY™ platform technology. We developed ARGX-119 through our IIP program in collaboration with the world leading key opinion leaders on MuSK and the neuromuscular junction, including Professor Steve Burden from NYU and Professor Verschuuren from LUMC. In collaboration with Professor Burden, it was shown that ARGX-119 holds promising preclinical POC data in Dok7 congenital myasthenic syndrome, observed in a mouse model bearing the most common patient mutation and in ALS using ALS patient derived NMJ on-a-chip models. Based on these data, clinical development for ARGX-119 was initiated as activation of MuSK by ARGX-119 may stabilize, mature, and improve the function of the NMJ in patients with CMS or ALS, significantly reducing weakness and fatigability and improving quality of life. A Phase 1 dose-escalation clinical trial in healthy volunteers started in 2023 and is ongoing. A Phase 1b and 2a clinical trial in CMS and ALS respectively are planned to start in 2024 to assess early signal detection in patients. ARGX-109, ARGX-220, ARGX-121 and ARGX-213 Development We continue to invest in our discovery engine, the IIP, to drive long-term sustainable pipeline growth. Through the IIP, four new pipeline candidates were nominated in 2023, including: ARGX-213 targeting FcRn and furthering argenx’ leadership in this new class of medicine; ARGX-121 and ARGX-220, which are first-in-class targets broadening argenx’ focus across the immune system; and ARGX-109, targeting IL-6, which plays an important role in inflammation. Preclinical work is ongoing for each candidate and we expect to file four IND applications by the end of 2025. Immunology Innovation Program Co-creation Through our IIP, we collaborate with scientific and academic partners to identify immunology breakthroughs and build potential pipeline candidates. For more information, please refer to section 1.1.2 “IIP”. Antibody Engineering and Other Technology Capabilities Our Proprietary SIMPLE ANTIBODY™ Platform Our proprietary SIMPLE ANTIBODY™ platform technology sources V-regions from conventional antibodies existing in the immune system of outbred llamas. Outbred llamas are those that have been bred from genetically diverse parents, and each has a different genetic background. The llama produces highly diverse panels of antibodies with a high human homology in their V-regions when immunized with human disease targets. We then combine these llama V-regions with Fc regions of fully human antibodies, resulting in antibodies that we then produce in industry-validated production cell lines. The resulting antibodies are diverse and, due to their similarity to human antibodies, we believe they are well suited to human therapeutic use. With this breadth of antibodies, we are able to test many different epitopes. Being able to test many different epitopes with our antibodies enables us to search for an optimized combination of safety, potency and species cross-reactivity with the potential for maximum therapeutic effect on disease. These antibodies are often cross-reactive with the rodent version of chosen disease targets. This rodent cross-reactivity enables more efficient preclinical development of our product candidates because most animal efficacy models are rodent-based. By contrast, most other antibody discovery platforms start with antibodies 1.3.6 1.3.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 ARGX-109, ARGX-220, ARGX-121 and ARGX-213 Development | 51 |
| 血液循環(pH 7.4) Cell Antibody Lysosome Endosome (pH 6.0) FcRN降解血清蛋白 圖4:FcRN介導的循環機制 在近交系小鼠或合成抗體庫中產生,我們認為這些方法分別受到抗體庫不足和多樣性有限的限制。我們簡單的 抗體™平臺技術允許我們訪問和探索廣泛的靶標,包括新的和複雜的靶標,同時最大限度地減少使用傳統方法生成候選抗體所需的長時間。 我們的抗體工程技術 通過許可,我們獲得了廣泛的抗體工程 技術。NHANCE™、ABDEG™、POTELIGENT®和DHS突變專注於 設計抗體的Fc區域,而SMART-Ig®和ACT-Ig®技術允許 製造全面抗體。 Fc工程可以增強抗體與免疫系統組件的相互作用, 從而通過改變抗體的半衰期、組織滲透率、疾病靶標清除率和效力來潛在地擴展我們候選產品的治療指數。例如,我們的NHANCE™和ABDEG™工程技術使我們能夠調節Fc區域與FcRN的相互作用,FcRN負責調節免疫球蛋白抗體的半衰期、組織分佈和 PD屬性。同樣,POTELLIGENT工程技術 調節抗體Fc區域與位於特殊免疫細胞(稱為自然殺傷(NK)細胞)上的受體之間的相互作用。這些NK細胞可以破壞靶細胞,導致增強的抗體依賴細胞介導的細胞毒性。 NHANCE™和ABDEG™: FcRN調節Fc與FcRN的相互作用。 FcRN介導的抗體循環機制如圖4所示。[1]血清蛋白,包括免疫球蛋白抗體,通常會被細胞攝取從循環中移除。[2]抗體可以與FcRN結合,FcRN是內體中專門的循環受體, 在酸性環境中, 然後[3A]通過與其Fc區域綁定到 FcRN,返回循環 。[3B]Unbound antibodies end up in the lysosomes and are degraded by enzymes. Because this Fc/FcRn interaction is highly pH-dependent, antibodies tightly bind to FcRn at acidic pH (pH 6.0) in the endosomes but release again at neutral pH (pH 7. 4) in the circulation. NHANCE™ NHANCE™ refers to two mutations that we introduce into the Fc region of an IgG antibody. NHANCE™ is designed to extend antibody serum half-life and increase tissue penetration. In certain cases, it is advantageous to engineer antibodies that remain in the circulation longer, allowing them to potentially exert a greater therapeutic effect or be dosed less frequently. As shown in Figure 5, [1]NHANCE ™抗體以更高的親和力與FcRn結合,特別是在酸性pH條件下。 [2]由於這些更緊密的鍵,NHANCE ™ FcRn介導的抗體 再循環強烈優於溶酶體降解,儘管確實發生了一些降解 。 [3]NHANCE™ allows a greater proportion of antibodies to return to the circulation ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Immunology Innovation Program | 52 |
| potentially resulting in increased bioavailability and reduced dosing frequency. ARGX-109, empasiprubart and a number of our discovery-stage programs utilize NHANCE™. Cell Antibody with NHance Lysosome Endosome FcRn Degraded serum proteins Figure 5: NHANCE™ mutations favor the FcRn-mediated recycling of IgG antibodies. Cell SIMPLE Antibody with ABDEG Lysosome Endosome FcRn Target Figure 6: SIMPLE ANTIBODY™ and ABDEG™ platform technologies work in concert to sweep diseases targets. ABDEG™ ABDEG™ refers to five mutations that we introduce in the Fc region that increase its affinity for FcRn at both neutral and acidic pH. In contrast to NHANCE™, ABDEG™-modified Fc regions remain bound to FcRn if the pH changes, occupying FcRn with such high affinity that they deprive endogenous IgG antibodies of their recycling mechanism, leading to enhanced clearance of such antibodies by the lysosomes. Some diseases mediated by IgG antibodies are directed against self-antigens. These self-directed antibodies are referred to as autoantibodies. We use our ABDEG™ technology to reduce the level of these pathogenic autoantibodies in the circulation by increasing the rate at which they are cleared by the lysosomes. ABDEG™ is a component in a number of our products and product candidates, including efgartigimod. As shown in Figure 6, our ABDEG™ technology can also be used with our pH-dependent SIMPLE ANTIBODY™ generated antibodies in a mechanism referred to as sweeping. Certain antibodies generated through the SIMPLE ANTIBODY™ platform bind to their target in a pH-dependent manner. These antibodies [1] 循環時,在中性pH值下與目標物緊密結合,以及 [2]在酸性pH值下釋放目標 [3]未結合的靶標在溶酶體中 降解。 [4]However, when equipped with our ABDEG™ technology, the therapeutic antibodies remain tightly bound to FcRn at all pH levels and are not degraded themselves. Instead, they are returned to the circulation where they can bind new targets. We believe this is especially useful in situations where high levels of the target are circulating or where the target needs to be cleared very quickly from the system. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Immunology Innovation Program | 53 |
| POTELLIGENT® POTELLIGENT® modulates the interaction of the Fc region with the Fc gamma receptor IIIa located on specialized immune cells, known as NK cells. These NK cells can destroy the target cell, resulting in enhanced ADCC. POTELLIGENT® changes the Fc structure by excluding a particular sugar unit such that it enables a tighter fit with the Fc gamma receptor IIIa. The strength of this interaction is a key factor in determining the killing potential of NK cells. An independent publication reported that the exclusion of this sugar unit of the Fc region increases the ADCC-mediated cell-killing potential of antibodies by 10- to 1000-fold. Cusatuzumab and ARGX-111 utilize POTELLIGENT® (source: Expert Opin Biol Ther 2006; 6:1161-1173; http://www.tandfonline.com/doi/full/10.1517/14712598.6.11.1161%20). SMART-Ig® , ACT-Ig® and DHS In 2020, we entered into a research license and option agreement with Chugai under which we may access Chugai’s SMART-Ig® and ACT-Ig® . In 2020, we also entered into a non-exclusive research agreement with the Clayton Foundation under which we may access the Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic antibodies. Genmab collaboration In 2023, we entered into a collaboration with Genmab to jointly discover, develop and commercialize novel therapeutic antibodies with applications in immunology, as well as in oncology therapeutic areas. The multiyear collaboration is expected to leverage the antibody engineering expertise and knowledge of disease biology of both companies to accelerate the identification and development of novel antibody therapeutic candidates with a goal to address unmet patient needs in immunology and cancer. Under the terms of the collaboration, argenx and Genmab each have access to the suites of proprietary antibody technologies of both companies to advance the identification of lead antibody candidates against differentiated disease targets. SC drug delivery technologies We have exclusive access to Halozyme’s ENHANZE® SC drug delivery technology for the FcRn and C2 targets and four additional targets. ENHANZE® has the potential to shorten drug administration time, reduce healthcare practitioner time, and offer additional flexibility and convenience for patients. In addition, in April 2021, we entered into the Elektrofi Agreement with Elektrofi to explore new SC formulations utilizing Elektrofi’s high concentration technology for efgartigimod, and up to one additional target. For more information on our collaborations, please refer to section 1.4 “Collaborations and licenses”. Partnered Programs See here for a description of collaboration and license agreements that we have entered into to further leverage our IIP. Collaborations and Licenses We follow a disciplined strategy to maximize the value of our pipeline. We plan to retain all development and commercialization rights to those products and product candidates that we believe we can commercialize successfully, if approved. 1.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 54 |
| We have partnered, and plan to continue to partner, to develop products and product candidates that we believe have promising utility in disease areas or have patient populations that may benefit from resources of other biopharmaceutical companies. We expect to continue to collaborate selectively with pharmaceutical and biotechnology companies to leverage our platform technology and accelerate product candidate development. We are also party to several license agreements under which we license patents, patent applications and other intellectual property to third parties. We have also entered into several license agreements under which we license patents, patent applications and other intellectual property from third parties. License agreements can relate to research and development and/ or commercialization of the relevant product candidates (and technologies) or products. The licensed intellectual property covers some of our product candidates and some of the antibody engineering technologies that we use. Some of these licenses impose various diligence and financial payment obligations on us. We expect to continue to enter into these types of license agreements in the future. We have entered into multiple collaboration agreements with pharmaceutical partners and license agreements, as described below. Our Strategic Collaboration with Shire In February 2012, we entered into a collaboration agreement with Shire AG (Shire, now known as Shire International GmbH) to discover, develop and commercialize novel human therapeutic antibodies against up to three targets to address diverse, rare and unmet diseases (Shire Collaboration Agreement). Pursuant to the Shire Collaboration Agreement, up through a specified period, we have granted Shire an exclusive option, against payment of a one-time option fee, to obtain all right, title and interest in any antibodies discovered under the collaboration. If Shire does not exercise its option with respect to any discovered antibody within a specified period, we are free to research, develop and commercialize antibodies in relation to the applicable clinical trial target, subject to negotiation of a license from Shire. OncoVerity for cusatuzumab In 2022, we, the University of Colorado Anschutz Medical Campus and the University of Colorado Health (UCHealth) created an asset-centric spin-off, OncoVerity, Inc. (OncoVerity) focused on optimizing and advancing the development of cusatuzumab, a novel anti-CD70 antibody, in AML. OncoVerity is an entity of co-creation, combining the extensive translational biology insights from Dr. Clayton Smith, M.D. from the University of Colorado with our experience on the CD70/CD27 pathway. In 2023, we granted an exclusive license for cusatuzumab to OncoVerity and provided, together with a joint venture of UCHealth and University License Equity Holdings, Inc. on the University of Colorado Anschutz Medical Campus, $26.0 million in funding for ongoing clinical development of cusatuzumab. Our Strategic Partnership with LEO Pharma for ARGX-112 (LP0145) In May 2015, we entered into a collaboration agreement with LEO Pharma A/S (LEO Pharma) to develop and commercialize ARGX-112 (LP0145) for the treatment of dermatologic indications involving inflammation (LEO Pharma Collaboration Agreement). ARGX-112 (LP0145) employs our SIMPLE ANTIBODY™ technology and blocks the IL-22R in order to neutralize the signaling of cytokines implicated in autoimmune diseases of the skin. LEO 1.4.1 1.4.2 1.4.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 55 |
| Pharma funded more than half of all product development costs up to clinical trial application (CTA) approval of a first product in a Phase 1 clinical trial, with our share of such costs capped, which was achieved in April 2018. Since then, LEO Pharma has been solely responsible for funding the clinical development of the program. In May 2021, CTA approval of a Phase 2a clinical trial for LP0145 was received. In September 2022, LEO Pharma, exercised its option to obtain, and was granted an exclusive, worldwide license to further develop and commercialize ARGX-112 against payment of a €5.0 million option fee to us. LEO Pharma assumed full responsibility for the continued development, manufacture and commercialization of such product and is subject to diligence obligations in respect of continuation of development and commercialization of such product. We are eligible to receive additional development, regulatory and commercial milestone payments in aggregate amount of up to €120.0 million, as well as tiered royalties on product sales at percentages ranging from the low single digits to the low teens, subject to customary reductions. Unless earlier terminated, the term of the LEO Pharma Collaboration Agreement ends upon the later of (i) the expiration of the last license granted under the agreement, and (ii) the fulfilment of all payment obligations under the agreement. LEO Pharma may terminate the LEO Pharma Collaboration Agreement for any reason upon prior written notice to us. LEO Pharma’s royalty payment obligations expire, on a product-by-product and country-by-country basis, upon the later of (i) a time when no valid claims covering such product, and (ii) (a) in major market countries with no composition of matter patent covering such product, the expiration of the data exclusivity period or (b) in countries that are not major market countries, a double-digit number of years after the first commercial sale of such product sold in that country. Our Strategic Partnership with Zai Lab for efgartigimod Pursuant to the Zai Lab Agreement, Zai Lab obtained the exclusive right to develop and commercialize efgartigimod in Greater China. Zai Lab will also contribute patients to our global Phase 3 clinical trials of efgartigimod. Additionally, the collaboration with Zai Lab is expected to accelerate efgartigimod global development by initiating multiple Phase 2 POC clinical trials in new autoimmune indications under our supervision; first indications for such POC clinical trials are kidney conditions LN and MN. Pursuant to the Zai Lab Agreement, we have received value worth $175.0 million from the Zai Lab Payments. We are also eligible to receive tiered royalties (mid-teen to low-twenties on a percentage basis) based on annual net sales of efgartigimod in Greater China. Our Strategic Partnership with AbbVie for ARGX-115 (ABBV-151) In April 2016, we entered into a collaboration agreement with AbbVie to develop and commercialize ARGX-115 (ABBV-151) as a cancer immunotherapy against the novel target glycoprotein A repetitions predominant (GARP) (the AbbVie Collaboration Agreement). ARGX-115 (ABBV-151) employs our SIMPLE ANTIBODY™ technology and works by stimulating a patient’s immune system after a tumor has suppressed the immune system by co-opting immunosuppressive cells such as regulatory T cells. Under the terms of the AbbVie Collaboration Agreement, we were responsible for conducting and funding all ARGX-115 (ABBV-151) research and development activities up to completion of IND enabling clinical trials. 1.4.4 1.4.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 56 |
| AbbVie已行使其選擇權並獲得了ARGX-115(ABBV-151)計劃的全球獨家許可證,以開發和商業化產品,並承擔了開發 義務,包括獨自承擔與基於ARGX-115(ABBV-151)的產品有關的所有研究、開發和監管費用 。根據AbbVie的 ARGX-115(ABBV-151)的持續進展,我們有資格分別獲得總計高達1.1億美元、1.9億美元和3.25億美元的開發、監管和 商業里程碑付款,以及按 從個位數中位數到十幾歲以下的百分比的產品銷售分級版税,但須按慣例減少。 根據AbbVie協作協議,我們有權根據副產品 ,在歐洲經濟區(EEA)和瑞士共同推廣基於ARGX-115(ABBV-151)的產品,並將該產品與我們自己的未來腫瘤學項目相結合(如果有)。共同推廣工作將由雙方本着 善意協商達成的共同推廣協議進行管理。 除非雙方因重大違約或AbbVie協作協議中另有規定而提前終止,否則許可協議的期限在履行協議下的所有付款義務 後終止,與ARGX-115(ABBV-151)計劃相關的 。 AbbVie可在事先書面通知我們的情況下,以任何理由終止AbbVie協作協議。AbbVie的版税支付義務將在下列時間中較晚的日期到期:(I)此類產品沒有有效的 索賠,(Ii)此類產品的監管或市場排他性終止,或(Iii)根據AbbVie合作協議在該 國家/地區銷售的此類產品首次商業銷售後10年。 我們與Elektrofi於2021年4月獲得的efgartigimod 獨家許可,我們與Elektrofi簽訂了Elektrofi協議,以利用Elektrofi用於efgartigimod的高濃度技術探索新的SC 配方,並最多可額外使用一個靶點。Elektrofi支持的配方旨在通過居家和自我管理功能為患者提供更多選擇。 根據Elektrofi協議的條款,我們在預定義的開發、監管和商業里程碑實現之前,對這兩個目標進行了預付款和未來 里程碑付款。Elektrofi還將從商業化產品的銷售中獲得個位數的版税。 我們與Genmab 在2023年進行了合作,我們與Genmab合作,共同發現、開發和 商業化新型治療性抗體,將其應用於免疫學以及腫瘤治療領域。這項多年的合作預計將利用兩家公司的抗體工程專業知識和疾病生物學知識,以加速 識別和開發新的抗體治療候選方案,目標是 滿足患者在免疫學和癌症方面的未得到滿足的需求。根據 合作條款,我們和Genmab各自可以使用兩家公司的專有抗體技術套件,以推進針對不同疾病目標的領先抗體候選的識別 。 1.4.6 1.4.7 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查聲明 財務非財務 信息 argenx 2023年年度報告合作和許可證|57 |
| Our Non-Exclusive Research License and Option Agreement with Chugai for SMART-Ig® and ACT-Ig® In September 2020, we entered into a non-exclusive research license and option agreement with Chugai, allowing us to access Chugai’s SMART-Ig® and ACT-Ig® engineering technologies for conducting feasibility clinical trials. These technologies are designed to enable us to make sweeping antibodies and expand the therapeutic index of our product candidates, which is the ratio between toxic and therapeutic dose, by potentially modifying their half‑life, tissue penetration, rate of disease target clearance and potency. Our Non-exclusive License with the Clayton Foundation for DHS mutations In October 2020, we entered into a non-exclusive research agreement with the Clayton Foundation relating to the non-exclusive in-license for Clayton Foundation’s proprietary DHS mutations to extend the serum half-life of therapeutic candidates. Our Exclusive License with Halozyme for ENHANZE® In February 2019, we entered into an in-license agreement with Halozyme for the use of certain patents, materials and know-how owned by Halozyme and relating to its ENHANZE®, for application in the field of prevention and treatment of human diseases (the ENHANZE ® License Agreement). Pursuant to the ENHANZE® License Agreement, we were granted exclusive rights to apply ENHANZE® to biologic products against pre-specified targets, in order to research, develop and commercialize SC formulations of our therapeutic antibody-based product candidates. Our first therapeutic target for which we received an exclusive license from Halozyme was FcRn, which allows us to apply ENHANZE® to efgartigimod and any other product candidates selective and specific for FcRn. Moreover, the breadth of our exclusive license to FcRn precludes either Halozyme itself or any of its current or future partners from utilizing ENHANZE® in the context of an FcRn-targeted product. Our second therapeutic target for which we received an exclusive license from Halozyme was human C2 associated with the product candidate empasiprubart, which is being developed to treat severe autoimmune diseases. Pursuant to the ENHANZE® License Agreement, we also have the right to nominate future targets for an exclusive ENHANZE® license if the target in question has not already been licensed by Halozyme or is not already being pursued by Halozyme. In October 2020, we expanded our collaboration with Halozyme for ENHANZE® drug delivery technology to include three additional exclusive targets upon nomination bringing the total to six potential targets. From the effective date of the ENHANZE® License Agreement, we have a seven-year period in which to conduct research and preclinical trials on other target-specific molecules in combination with ENHANZE® and may nominate up to four additional targets we have not yet nominated for an exclusive commercial license. Pursuant to the ENHANZE® License Agreement, we have the right to grant sublicenses to our subsidiaries and to third parties both for research/preclinical work (for example, to subcontractors) and for development and commercialization. Halozyme provides dedicated specialist support to us which it has accrued over 10 years of licensing ENHANZE® to its collaborators. 1.4.8 1.4.9 1.4.10 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 58 |
| Upon nomination of any future target for an exclusive commercialization license and confirmation by Halozyme that such a license is available, we will pay $12.5 million to Halozyme per target. We will be obligated to pay clinical development, regulatory and commercial milestones totaling $160.0 million for the first product that uses ENHANZE® and is specific for a given target. We are also obligated to pay Halozyme a percentage of net sales as a royalty of any licensed product that uses ENHANZE®. This royalty varies with net sales volume, ranging from the low to mid-single digits. The royalty obligations may be reduced by up to 50% under different circumstances including the requirement of a compulsory license from a government, the need to secure third-party licenses to enable sales of our products using the ENHANZE® technology, and/or the lack of patent coverage in a particular country. We have diligence obligations with respect to the continuation of development and commercialization of product candidates, but we are not obligated to utilize ENHANZE® for every product candidate directed to a given exclusive target(s). We may terminate the ENHANZE® License Agreement at any time, either in its entirety or on a target-by-target basis, by sending Halozyme prior written notice. Absent early termination, the ENHANZE® License Agreement will automatically expire upon the expiry of our royalty payment obligations under the agreement. In the event the ENHANZE® License Agreement is terminated for any reason, the license granted to us would terminate but Halozyme would grant our sublicensees a direct license following such termination. In the event the ENHANZE® License Agreement is terminated other than for our breach, we would retain the right to sell licensed products then on hand for a certain period of time post-termination. As also set out in section “Corporate Governance”, our non-executive director James M. Daly previously served as a non-executive member of the board of directors of Halozyme. Mr. Daly did not participate in any discussions and decision making relating to the ENHANZE® License Agreement. The ENHANZE® License Agreement with Halozyme was not a related party transaction in accordance with IAS 24 - Related Party Disclosures, since Mr. Daly, in his role as non-executive director, did not control or have significant influence over argenx or Halozyme. However, the ENHANZE® License Agreement does constitute a related party transaction under the applicable SEC rules and is therefore reported as such our 2023 20-F “Related Party Transactions”. Our Exclusive License with Agomab for ARGX-114 (AGMB-101) In March 2019, we entered into an exclusive out-license with Agomab for the use of certain patent rights relating to our proprietary suite of technologies for the development and commercialization of a series of agonistic anti-MET SIMPLE ANTIBODY™ generated antibodies, including ARGX-114 (AGMB-101), a halofuginone-mimetic antibody directed against the MET receptor. Agomab is required to use commercially reasonable efforts to develop and commercialize at least one licensed product. In connection with our entry into this agreement, we received a profit-sharing certificate which entitles us to 20% of all distributions to Agomab’s shareholders (which shall be reduced to 10% following the filing of an IND and is subject to further adjustment upon the occurrence of certain financings). Upon the occurrence of a qualified initial public offering of Agomab, the profit-sharing certificate will automatically be converted into the equivalent number of ordinary shares in Agomab. This agreement is subject to mutual termination for material breach or insolvency and automatically expires upon the expiration of the last to expire of our licensed patent rights. 1.4.11 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 59 |
| Our Exclusive License with Broteio for empasiprubart In March 2017, we entered into a collaboration with Broteio in connection with our IIP, to develop an antibody against a novel target in the complement cascade, empasiprubart (Broteio Agreement). Under the Broteio Agreement, we are jointly developing the complement-targeted antibody to seek to establish preclinical POC using our proprietary suite of technologies. Upon successful completion of these clinical trials, we exercised an exclusive option to in-license the program in March 2018 and assumed responsibility for further development and commercialization. Pursuant to the Broteio Agreement, we are obligated to make milestone payments upon the occurrence of certain development milestones (up to an aggregate of €4.0 million), commercialization milestones (up to an aggregate of €10.0 million) and pay tiered royalties on net sales in the low single digits. We may terminate the Broteio Agreement for convenience upon 90 days prior written notice. The Broteio Agreement is also subject to mutual termination for material breach or insolvency and automatically expires upon the expiration of our financial obligations thereunder. Our Exclusive License with VIB for ARGX-118 In November 2016, we entered into a collaboration under our IIP with VIB vzw (VIB) to develop antibodies against Galectin-10, the protein of Charcot-Ley-den Crystals, which play a major role in severe asthma and the persistence of mucus plugs, including ARGX-118 (VIB Agreement). Pursuant to the VIB Agreement, we are jointly developing antibodies against Galectin-10 using our proprietary suite of technologies. Upon successful completion of this initial research, we exercised an exclusive option to in-license the program and assumed responsibility for further development and commercialization. Under the VIB Agreement, including as amended in November 2018, we are obligated to make milestone payments upon the occurrence of certain development milestones (up to an aggregate of €4.0 million), commercialization milestones (up to an aggregate of €11.0 million) and pay tiered royalties on net sales in the low single digits. We may terminate the VIB Agreement for convenience upon 90 days prior written notice. The VIB Agreement is also subject to mutual termination for material breach, insolvency or certain patent challenges and automatically expires upon the expiration of VIB’s licensed patent rights. Our Exclusive License with the University of Texas for NHANCE™ and ABDEG™ In February 2012, we entered into an exclusive in-license with the Board of Regents of the University of Texas System (UT BoR) for the use of certain patent rights relating to the NHANCE™ platform for any use worldwide (the UT Agreement). The UT Agreement was amended on December 23, 2014 to also include certain additional patent rights relating to the ABDEG™ platform. Upon commercialization of any of our products that use the in-licensed patent rights, we will be obligated to pay UT BoR a percentage of net sales as a royalty until the expiration of any patents covering the product. This royalty varies with net sales volume and is subject to an adjustment for royalties we receive from a sublicensee of our rights under the UT Agreement, but in any event does not exceed 1%. In addition, we must make annual license maintenance payments to UT BoR until termination of the UT Agreement and we have assumed certain development and commercial milestone payment and reimbursement obligations. We also have diligence requirements with respect to development and commercialization of products which use the in-licensed patent rights. 1.4.12 1.4.13 1.4.14 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Collaborations and Licenses | 60 |
| 根據UT協議,我們可以向第三方授予再許可。如果我們收到與此類再許可相關的任何 非特許權使用費收入,我們必須根據再許可的性質向UT BOR支付從中低個位數到中上個位數不等的百分比的 此類收入。如果再被許可人同意支付UT協議中規定的里程碑式付款,則免除此類費用。 為方便起見,我們可以事先書面通知單方面終止UT協議。 如果不提前終止,UT協議將在 UT協議涵蓋的專利權範圍內的所有已頒發專利和提交的專利申請到期時自動失效。我們的特許權使用費支付義務在沒有有效索賠的情況下按產品和國家/地區到期。 我們與BioWa的非獨家許可以及 與BioWa和Lonza的非獨家商業許可 2010年10月,我們與BioWa,Inc.(BioWa) 就使用BioWa擁有的、與其POTELLIGENT®平臺技術相關的某些專利和專有技術簽訂了非獨家許可協議,用於預防和治療人類疾病領域(《生物圈協定》)。根據BioWa協議,我們被授予使用POTELLIGENT®研究和開發POTELLIGENT®的抗體和含有此類抗體的產品的非獨家權利。 2013年和2014年,我們與BioWa和龍沙簽訂了POTELLIGENT®CHOK1SV的非獨家許可協議,以分別進一步開發、製造和商業化POTELLIGENT®-110和ARGX-111(POTELLIGENT ®許可協議)。 在我們使用POTELLIGENT ARGX開發的產品商業化後,我們將有義務 向BioWa和Lonza支付許可產品淨銷售額的一定百分比作為版税。此 使用費隨淨銷售量的不同而不同,範圍在較低的個位數之間,如果 自產品在一個國家/地區的首次商業銷售起計的10年內 該產品所涵蓋的許可專利(S)內的最後一個有效權利要求到期或終止,則版税將減半。此外,我們還必須每年支付研究許可證維護費,該費用將在我們向BioWa支付的版税開始時停止。對於產品的持續開發和商業化,我們對 有勤勉要求。我們還承擔了某些開發、監管和商業里程碑付款義務, 必須每年報告我們在實現這些里程碑方面的進展。 BioWa的里程碑將按商業目標逐個支付,如果我們實現全球年銷售額超過10億美元,我們有義務為每個商業目標支付總計高達3,600萬美元的里程碑付款。 根據POTELLIGENT®許可協議,我們有權向 第三方授予再許可。BioWa保留獨家談判權,僅在某些國家/地區開發我們使用POTELLIGENT®開發的產品並將其商業化。 我們可以通過發送BIOWA和LONZA事先書面通知隨時終止POTELLIGENT®許可協議。如果不提前終止,POTELLIGENT®許可協議 將在我們根據POTELLIGENT®許可協議承擔的版税義務到期時自動到期。如果POTELLIGENT®許可協議因任何 原因終止,授予我們的許可將終止,但BioWa將在終止後授予我們的分許可人 直接許可。如果簽訂了POTELLIGENT資本許可協議 1.4.15 ar Gr g oup enx Factors Risk Go Corporate vernance® 共享財務 審核聲明 財務非財務 信息 Argenx年度報告2023年協作和許可|61 |
| 如果非因違約或破產而終止,我們將保留在終止後的一段時間內銷售當時手頭的許可產品的權利。 我們於2015年2月4日與Lonza簽訂了 多產品GS Xceed-許可 的非獨家許可,我們與龍沙簽訂了一項非獨家多產品內許可協議,使用龍沙專有的谷氨醯胺合成酶基因 表達系統GS Xceed™,該系統由中國倉鼠卵巢細胞系和用於製造藥物的載體(該系統)的 載體組成。該系統用於製造Efgartigimod、Empasiprubart和ARGX-119等。 根據多產品許可證,我們有權向某些預先批准的第三方授予再許可,而無需事先獲得Lonza的書面同意,但否則我們必須獲得Lonza的事先書面同意。 我們已對Lonza承擔了某些開發、監管和商業里程碑付款 義務。我們被要求使用該系統支付這些里程碑。我們有義務向龍沙支付開發、監管和商業里程碑付款。 我們可以通過事先書面通知龍沙,逐個產品終止多產品許可證。龍沙可能僅在違約或發生資不抵債事件時終止多產品許可證。如果不提前終止,多產品許可證將在該產品的最後一個有效申請到期時自動 過期。我們或我們的戰略合作伙伴 將保留在終止後銷售各自產品的權利。 我們與天主教大學(UCL)和Sopartec S.A.(Sopartec) 在2013年1月就GARP 與UCL及其技術轉移公司Sopartec簽訂了合作和獨家產品許可協議,以發現和開發針對GARP的新型人類 治療性抗體(GARP協議)。根據《GARP協議》, 各方根據共同商定的研究計劃,承擔與分配給它的活動相關的所有費用。 2015年1月,我們行使了根據《GARP協議》授予我們的選擇權, 簽訂了獨家的全球商業內部許可,以使用UCL和路德維希癌症研究所擁有的某些與GARP相關的知識產權,以進一步開發和商業化許可產品,包括根據原始合作協議(GARP許可證)發現的GARP中和抗體ARGX-115。 在GARP協議到期後,GARP許可證將成為GARP知識產權項下用於任何目的的全額已付清、 永久全球獨家許可證, 受UCL保留非商業性研究權利的約束。 根據GARP許可證,我們可以向此類 第三方及其附屬公司授予再許可。2016年,我們與 AbbVie簽訂了關於ARGX-115的獨家協作和許可協議。從我們收到的與這些 子許可相關的任何收入中,例如與AbbVie協作協議相關的收入,我們必須向Sopartec支付該收入的較低十位數範圍的百分比。特許權使用費支付 如果沒有 1.4.16 1.4.17 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 argenx年度報告2023年協作和許可證|62,則按產品和國家/地區到期 |
| valid claims covering the ARGX-115 product. We also have diligence obligations with respect to the continued development and commercialization of ARGX-115 products. Our Exclusive License with NYU Langone Health and LUMC for ARGX-119 In 2019 and 2020, we entered into collaboration and exclusive license agreements with NYU Langone Health and LUMC under our IIP to develop antibodies targeting the MuSK, for the treatment neuromuscular diseases, which play a major role at the neuromuscular junction (NYU and LUMC Agreements). Pursuant to the NYU and LUMC Agreements, we, NYU and LUMC jointly developed antibodies against MuSK using our proprietary suite of technologies. Under the NYU and LUMC Agreements, as amended, we are obligated to make milestone payments upon the occurrence of certain development milestones, commercialization milestones and pay tiered royalties on net sales in the low single digits. Distribution Agreements We are parties to the Medison Agreement, the Medison Multi-Regional Agreement, Genpharm Agreement, and the Handok Agreement. Manufacturing and Supply We utilize third-party contract manufacturers who act in accordance with the FDA’s current good manufacturing practices (cGMPs) for the manufacture of drug substance and drug product. We continue to build our global network of contract manufacturers to support the development and commercialization of our products. We work with Lonza teams based in Slough, UK, Portsmouth, U.S., Singapore and Visp, Switzerland for activities relating to the development of cell banks, development of our manufacturing processes and the manufacturing of drug substance, thereby using validated and scalable systems broadly accepted in our industry. In 2022, we started our collaboration with FUJIFILM Diosynth Biotechnologies Denmark ApS (Fujifilm) based in Hillerød, Denmark, for activities relating to the large-scale manufacturing of efgartigimod drug substance. We use additional contract manufacturers to fill, label, package, store and distribute (investigational) drug products. Intellectual Property Introduction We strive to protect and maintain exclusivity for the proprietary technologies that we believe are important to our business, patients and shareholders. We are focused on pursuing and maintaining patent protection intended to cover core platform technologies incorporated into, or used to produce, our product candidates and commercial products. We will seek protection for our innovations in key global jurisdictions. We continue to focus our exclusivity strategies on all aspects of our assets, including our compositions of matter, methods of use for our approved products, and other inventions that are important to our business (e.g., the patient innovations described in our product labels/product inserts and our core manufacturing technologies). 1.4.18 1.5 1.6 1.7 1.7.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Distribution Agreements | 63 |
| Our intellectual property portfolio continues to grow and keep pace with the innovations arising from our discovery, development, and commercial efforts. We expect the total volume of patent positions under our management to increase with each year as our pipeline evolves. We currently oversee more than 500 pending applications and granted patents. More importantly, as we continue to innovate for patients, we will work to protect our patient innovations with new intellectual property filings to enable future reinvestment for patients. In addition to patent protection, we rely on trademarks and trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of our llama immunization and antibody affinity maturation approaches. Our commercial success depends in part upon our ability to obtain and maintain exclusivity, including regulatory exclusivities, patent, and other proprietary protection for commercially important technologies, inventions and know-how related to our business. We will defend and enforce our intellectual property rights, particularly our patent rights, and preserve the confidentiality of our trade secrets while operating without infringing valid and enforceable intellectual property rights of others. Specifically, we are materially dependent on regulatory, patent and other proprietary protection related to our core platform technologies, described in section “Platform Technologies”, and our product candidates, as described in section “Our Internal Programs” and section “Our Partnered Programs”. The patent positions for biotechnology companies like us are generally uncertain and can involve complex legal, scientific and factual issues. In addition, the coverage claimed in a patent application can be significantly reduced before a patent is issued, and its scope can be reinterpreted and even challenged after issuance. As a result, we cannot guarantee that any of our platform technologies and product candidates will be protectable or remain protected by enforceable patents. We cannot predict whether the patent applications we are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will provide sufficient proprietary protection from competitors. Any patents that we hold may be challenged, circumvented or invalidated by third parties. The term of individual patents depends upon the legal term of the patents in the countries in which they are obtained. In most countries in which we file, the patent term is 20 years from the earliest date of filing a non-provisional patent application. In the U.S., the term of a patent covering an FDA-approved drug may be eligible for a limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Act) as compensation for the loss of patent term during the FDA regulatory review process as described in section “Licensure and Regulation of Biologics in the U.S.”. Similar provisions are available in the EU and in certain other jurisdictions to extend the term of a patent that covers an approved drug. It is possible that issued U.S. patents covering each of our product candidates may be entitled to patent term extensions. If our product candidates receive FDA approval, we intend to apply for patent term extensions, if available, to extend the term of patents that cover the approved product candidates. We also intend to seek patent term extensions in any jurisdictions where they are available, however, there is no guarantee that the applicable authorities, including the FDA, will agree with our assessment of whether such extensions should be granted, and if granted, the length of such extensions. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 64 |
| Platform Technologies With regard to our platform technologies, we own or have intellectual property rights directed to our SIMPLE ANTIBODY™ discovery platform, the ABDEG™ and NHANCE™ technologies. With regard to our SIMPLE ANTIBODY™ discovery platform, we have a broad patent portfolio providing exclusivity on the SIMPLE ANTIBODY™ platform. We expect to enjoy exclusivity under this patent portfolio until between 2029 and 2033. With regard to the ABDEG™ platform, we co-own the technology with UT Southwestern and enjoy certain exclusive license rights. We have a broad patent portfolio covering the composition of matter and uses of certain FcRn antagonists to achieve certain biological effects. The composition of matter and other relevant patents in the U.S. expire in 2036 whereas in many other countries the base expiry date is 2034. With regard to the NHANCE™ platform, we exclusively licensed two U.S. patents from UT Southwestern with composition of matter claims directed to an IgG molecule comprising a variant human Fc domain, and method of use claims directed to a method of blocking FcRn function in a subject by providing to the subject such an IgG molecule. The U.S. patents are expected to expire earliest in 2027 to 2028. The patent family also includes a granted European patent. Our Internal Programs Efgartigimod Efgartigimod incorporates the ABDEG™ platform technology. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Our ARGX-109 Product Candidate With regard to our wholly-owned ARGX-109 product candidate, we have one patent family with composition of matter claims directed to ARGX-109. The patent family has a basic expiry date in 2033. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Furthermore, ARGX-109 incorporates or employs the SIMPLE ANTIBODY™ platform technology and the NHANCE™ platform technology. Empasiprubart Product Candidate With regard to the empasiprubart product candidate, we own or have rights to multiple patent families (including one in-licensed patent family from Broteio) with several granted patents and pending patent applications in multiple jurisdictions in North America, South America, the EU and Asia, directed to composition of matter claims and method of treatment claims. The in-licensed patent family from Broteio has granted patents in several countries/regions and has a basic expiry date in 2034. Additional patent families have granted patents with basic expiry dates in 2039 and 2040. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. empasiprubart product candidate incorporates or employs the NHANCE™ platform technology. 1.7.2 1.7.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 65 |
| Our ARGX-119 Product Candidate With regard to the ARGX-119 product candidate, we in-licensed patent families from/with NYU Langone Health, a U.S. medical center based in New York, and additional patent families from/ with the LUMC, with a U.S. granted patent and several pending applications in multiple jurisdictions. We anticipate several more patient innovations to evolve during development for which we will seek additional patent protection. Our ARGX-118 Product Candidate With regard to the ARGX-118 product candidate, we co-own a patent portfolio with VIB, an inflammation research center in Ghent, Brussels, and Ghent University, with one U.S. granted patent and pending patent applications in multiple jurisdictions in North America, South America, the EU and Asia. The patent family has a basic expiry date in 2039. Our Partnered Programs Our Cusatuzumab (ARGX-110) Product Candidate With regard to the cusatuzumab product candidate, we have a broad patent portfolio that include claims to the composition of matter, uses of the molecule, and other important inventions. The issued U.S. patents expire in 2032 and 2033, without taking a potential patent term extension into account. Cusatuzumab incorporates or employs the SIMPLE ANTIBODY™ and POTELLIGENT® platform technologies. Our ARGX-115 (ABBV-151) Product Candidate With regard to the ARGX-115 (ABBV-151) product candidate that we co-own with, and exclusively license from, the Ludwig Institute for Cancer Research and UCL, we have a patent portfolio that includes a U.S. patent with a basic expiry date in 2034, without taking a potential patent term extension into account. There is a second family with meaningful patent coverage to the composition of matter and epitope claims that are expected to expire in 2036 and 2038. Furthermore, ARGX-115 (ABBV-151) incorporates or employs the SIMPLE ANTIBODY™ platform technology. Our ARGX-112 (LP-0145) Product Candidate With regard to the ARGX-112 (LP-0145) product candidate, we have one patent family with composition of matter claims directed to an antibody that binds human IL-22R. The patent family has a basic expiry date in 2037. Furthermore, ARGX-112 (LP-0145) incorporates the SIMPLE ANTIBODY™ platform technology. Trade Secret Protection In addition to patent protection, we also rely on trade secret protection to ensure exclusivity for our proprietary information that is not amenable to, or that we do not consider appropriate for, patent protection, including, for example, certain aspects of our llama immunization and antibody affinity maturation approaches. However, trade secrets can be difficult to protect. Although we take steps to protect our proprietary information, including restricting access to our premises and our confidential information, as well as entering into agreements with our employees, consultants, advisors and potential collaborators, third parties may independently develop the same or similar proprietary information or may otherwise gain access to our proprietary information. As a result, we may be unable to meaningfully protect our trade secrets and proprietary information. 1.7.4 1.7.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 66 |
| Regulation Government authorities in the U.S., at the federal, state and local level, and in the EU and other countries and jurisdictions, extensively regulate, among other things, the research, development, testing, manufacture, quality control, approval, packaging, storage, recordkeeping, labeling, advertising, promotion, distribution, marketing, post-approval monitoring and reporting, and import and export of pharmaceutical products, including biological products. In addition, some jurisdictions regulate the pricing of pharmaceutical products. The processes for obtaining marketing approvals in the U.S. and in other countries and jurisdictions, along with subsequent compliance with applicable statutes and regulations and other regulatory authorities, require the expenditure of substantial time and financial resources. Licensure and Regulation of Biologics in the U.S. In the U.S., biological products used for the prevention, treatment, or cure of a disease or condition in a human being are subject to regulation under the U.S. Federal Food, Drug, and Cosmetic Act (FDCA) and its implementing regulations, with the exception that the section of the FDCA that governs the approval of drugs via NDAs does not apply to the approval of biologics. Biologics are approved for marketing under provisions of the Public Health Service Act (PHSA) via biologics license applications (BLAs). However, the application process and requirements for approval of BLAs are very similar to those for new drug applications (NDAs). The failure to comply with the applicable U.S. requirements at any time during the product development process, including preclinical testing and clinical testing, the approval process or post-approval process may subject an applicant to delays in the conduct of a clinical trial, regulatory review and approval, and/or administrative or judicial sanctions. These sanctions may include, but are not limited to, the FDA’s refusal to allow an applicant to proceed with clinical testing, refusal to approve pending applications, license suspension or revocation, warning or untitled letters, adverse publicity, product recalls, product seizures, total or partial suspension of production or distribution, injunctions, fines and civil or criminal investigations and penalties brought by the FDA or the Department of Justice or other governmental entities. An applicant seeking approval to market and distribute a new biologic in the U.S. generally must satisfactorily complete each of the following steps: · preclinical laboratory tests, animal studies and formulation studies all performed in accordance with applicable regulations, including the GLPs; · submission to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin; · approval by an institutional review board (IRB) representing each clinical site before each clinical trial may be initiated; · performance of adequate and well-controlled human clinical trials to establish the safety, potency and purity of the product candidate for each proposed indication, in accordance with good clinical practices (GCPs); · preparation and submission to the FDA of a BLA for a biological product requesting marketing for one or more proposed indications, including submission of detailed information on the manufacture and composition of the product in clinical development and proposed labeling; 1.8 1.8.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation | 67 |
| · one or more FDA inspections of the manufacturing facility or facilities, including those of third parties, at which the product, or components thereof, are produced to assess compliance with cGMP requirements and to assure that the facilities, methods and controls are adequate to preserve the product’s identity, strength, quality and purity; · FDA audits of the clinical trial sites to assure compliance with GCPs, and the integrity of clinical data in support of the BLA; · payment of user fees and securing FDA approval of the BLA and licensure of the new biological product; and · compliance with any post-approval requirements, including the potential requirement to implement a risk evaluation and mitigation strategy (REMS) and any post-approval studies required by the FDA; and preclinical trials and INDs. Before testing any biological product candidate in humans, the product candidate must undergo preclinical testing. Preclinical tests include laboratory evaluations of product chemistry, formulation and stability, as well as animal studies to evaluate the potential for activity and toxicity. The conduct of the preclinical tests and formulation of the compounds for testing must comply with federal regulations and requirements. The results of the preclinical tests, together with manufacturing information and analytical data, are submitted to the FDA as part of an IND application. The IND automatically becomes effective 30 days after receipt by the FDA, unless before that time the FDA raises concerns or questions about the product candidate or conduct of the proposed clinical trial, including concerns that human research subjects will be exposed to unreasonable health risks, and places the proposed clinical trial on clinical hold. In that case, the IND sponsor and the FDA must resolve any outstanding FDA concerns before the clinical trial can begin. As a result, submission of the IND may result in the FDA not allowing the clinical trial to commence or on the terms originally specified by the sponsor in the IND. If the FDA imposes a partial or complete clinical hold, this action would delay either a proposed clinical trial or cause suspension of an ongoing clinical trial, or in the case of a partial clinical hold place limitations on the conduct of the clinical trial such as duration of treatment, until all outstanding concerns have been adequately addressed and the FDA has notified the company that investigation may proceed and then only under terms authorized by the FDA. This could cause significant delays or difficulties in completing planned clinical trials in a timely manner. The FDA may impose clinical holds on a biological product candidate at any time before or during clinical trials due to safety concerns or non-compliance. Human Clinical Trials in Support of a BLA Clinical trials involve the administration of the investigational product candidate to healthy volunteers or patients with the disease to be treated under the supervision of a qualified principal investigator in accordance with GCPs. Clinical trials are conducted under clinical trial protocols detailing, among other things, the objectives of the clinical trial, inclusion and exclusion criteria, the parameters to be used in monitoring safety, and the effectiveness criteria to be evaluated. A protocol for each clinical trial and any subsequent protocol amendments must be submitted to the FDA as part of the IND. A sponsor who wishes to conduct a clinical trial outside the U.S. may, but is not required to, obtain FDA clearance to conduct the clinical trial under an effective IND. If a foreign clinical trial is not conducted under an IND, the sponsor may submit data from the clinical trial to the FDA in support of the BLA so long as the clinical trial is well-designed and well-conducted in accordance with GCPs, including review and approval by an independent ethics committee, and the FDA is able to validate the clinical trial data through an onsite inspection, if necessary. Further, each clinical trial must be reviewed and approved by the IRB or, if applicable, the Ethics Committee, either centrally or individually at each institution at which the clinical trial ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 68 |
| will be conducted. The IRB or the Ethics Committee will consider, among other things, clinical trial design, patient informed consent, ethical factors and the safety of human subjects. An IRB must operate in compliance with FDA regulations. The FDA, IRB, the Ethics Committee or the clinical trial sponsor may suspend or discontinue a clinical trial at any time for various reasons, including a finding that the clinical trial is not being conducted in accordance with FDA requirements or the subjects or patients are being exposed to an unacceptable health risk. Clinical testing also must satisfy extensive GCPs and the requirements for informed consent. Additionally, some clinical trials are overseen by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring board or committee. This group may recommend continuation of the clinical trial as planned, changes in clinical trial conduct, or cessation of the clinical trial at designated check points based on access to certain data from the clinical trial. Clinical trials typically are conducted in three sequential phases, but the phases may overlap or be combined. Additional clinical trials may be required after approval. · Phase 1 clinical trials are initially conducted in a limited population to test the product candidate for safety, including adverse effects, dose tolerance, absorption, metabolism, distribution, excretion and PD in healthy humans or, on occasion, in patients, such as cancer patients. · Phase 2 POC clinical trials are generally conducted in a limited patient population to identify possible adverse effects and safety risks, evaluate the efficacy of the product candidate for specific targeted indications and determine dose tolerance and optimal dosage. Multiple Phase 2 clinical trials POC may be conducted by the sponsor to obtain information prior to beginning larger Phase 3 clinical trials. · Phase 3 clinical trials proceed if the Phase 2 POC clinical trials demonstrate that a dose range of the product candidate is potentially effective and has an acceptable safety profile. Phase 3 clinical trials are undertaken within an expanded patient population to gather additional information about safety and effectiveness necessary to evaluate the overall benefit-risk relationship of the drug and to provide an adequate basis for physician labeling. In some cases, the FDA may approve a BLA for a product candidate but require the sponsor to conduct additional clinical trials to further assess the product candidate’s safety and effectiveness after approval. Such post-approval clinical trials are typically referred to as Phase 4 clinical trials. These clinical trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and to document a clinical benefit in the case of biologics approved under accelerated approval regulations. If the FDA approves a product while a company has ongoing clinical trials that were not necessary for approval, a company may be able to use the data from these clinical trials to meet all or part of any Phase 4 clinical trial requirement or to request a change in the product labeling. Failure to exhibit due diligence with regard to conducting required Phase 4 clinical trials could result in withdrawal of approval for products. Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and the investigators 15 days after the clinical trial sponsor determines the information qualifies for reporting for serious and unexpected suspected adverse events, findings from other clinical trials or animal or in vitro testing that suggest a significant risk for human subjects and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure. The sponsor must also notify the FDA of any unexpected fatal or life-threatening suspected adverse reaction as soon as possible but in no case later than seven calendar days after the sponsor’s initial receipt of the information. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 69 |
| A drug being studied in clinical trials may be made available to individual patients in certain circumstances. Pursuant to the 21st Century Cures Act, as amended, the manufacturer of an investigational drug for a serious disease or condition is required to make available, such as by posting on its website, its policy on evaluating and responding to requests for individual patient access to such investigational drug. This requirement applies on the earlier of the first initiation of a Phase 2 POC or Phase 3 clinical trial of the investigational drug, or as applicable, 15 days after the drug receives a designation as a breakthrough therapy, fast track product, or regenerative advanced therapy. Compliance with cGMP Requirements Before approving a BLA, the FDA typically will inspect the facility or facilities where the product is manufactured. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. The PHSA emphasizes the importance of manufacturing control for products like biologics whose attributes cannot be precisely defined. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, the sponsor must develop methods for testing the identity, strength, quality, potency, and purity of the final biological product. Additionally, appropriate packaging must be selected and tested, and stability studies must be conducted to demonstrate that the biological product candidate does not undergo unacceptable deterioration over its shelf life. Manufacturers and others involved in the manufacture and distribution of products must also register their establishments with the FDA and certain state agencies. Both domestic and foreign manufacturing establishments must register and provide additional information to the FDA upon their initial participation in the manufacturing process. Establishments may be subject to periodic unannounced inspections by government authorities to ensure compliance with the FDCA, cGMPs and other requirements. Manufacturers may have to provide, on request, electronic or physical records regarding their establishments. Disclosure of Clinical Trial Information Sponsors of clinical trials of FDA-regulated products, including biological products, are required to register and disclose certain clinical trial information on the website www.clinicaltrials.gov. Information related to the product, patient population, phase of investigation, clinical trial sites and investigators, and other aspects of a clinical trial are then made public as part of the registration. Sponsors are also obligated to disclose the results of their clinical trials after completion. Disclosure of the results of clinical trials can be delayed in certain circumstances for up to two years after the date of completion of the clinical trial. Competitors may use this publicly available information to gain knowledge regarding the progress of clinical development programs as well as clinical trial design. Review and Approval of a BLA The results of product candidate development, preclinical testing and clinical trials, including negative or ambiguous results as well as positive findings, are submitted to the FDA as part of a BLA requesting a license to market the product. The BLA must contain extensive manufacturing information and detailed information on the composition of the product and proposed labeling as well as payment of a user fee. The FDA has 60 days after submission of the application to conduct an initial review to determine whether the BLA is sufficient to file based on the agency’s threshold determination that it is sufficiently complete to permit substantive review. If the FDA determines the BLA is not sufficiently complete, it will refuse to file the BLA. Once the submission has been filed, the FDA begins an in-depth review of the application. Under the goals agreed to by the FDA under ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 70 |
| 對於PDUFA,FDA有10個月的時間完成對標準申請的初步審查並答覆申請人,如果沒有根據該計劃提交BLA,則有6個月的時間完成對申請的優先審查。如果BLA是根據該計劃提交的,則在審查時鐘中再增加兩個月,無論是標準審查還是優先審查,總審查時間分別為12個月或8個月。FDA 並不總是達到其PDUFA標準審查和優先審查的目標日期。審查流程和PDUFA目標日期也可以延長三個月,如果FDA提出要求,或者如果申請人以其他方式提供關於提交中已經提供的信息的補充信息或澄清,可以被視為 重要信息。 在FDA對申請和附帶信息進行評估後,包括對生產設施的任何潛在檢查的結果和FDA對臨牀試驗地點的任何審計的結果,以確保符合GCP,FDA將出具批准信或 完整的回覆信。批准函授權該產品的商業營銷,並提供特定適應症的具體處方信息。根據PHSA,如果FDA確定產品是安全、純淨和有效的,並且將生產產品的設施符合旨在確保產品繼續安全、純淨和有效的標準,則FDA可以 批准BLA。如果申請未獲批准,FDA將發出完整的 回覆信,其中將確定申請中的不足之處以及為確保申請獲得批准而必須滿足的條件,並在可能的情況下概述贊助商可能採取的為獲得申請批准而可能採取的建議行動。收到完整回覆信的贊助商 可以向FDA提交代表對FDA確定的問題的完整回覆的信息,撤回申請或請求舉行 聽證會。在完整的回覆信中確定的問題得到解決之前,FDA不會批准申請。 FDA還可以將申請提交給諮詢委員會進行審查、評估和 是否應批准申請的建議。特別是,FDA可能會將新型生物製品或提出安全性或有效性難題的生物製品的申請提交給諮詢委員會。通常,諮詢委員會是由包括臨牀醫生和其他科學專家在內的獨立專家組成的 小組,負責審查、評估申請,並就申請是否應獲得批准以及在何種條件下批准提出建議。FDA不受諮詢委員會的建議的約束,但它在做出決定時會仔細考慮這些建議。 如果FDA批准了一種新產品,它可能會限制該產品的批准適應症。它還可能要求在產品標籤中包含禁忌症、警告或預防措施。此外,FDA可能會要求進行批准後的研究,包括4期臨牀試驗,以進一步評估該產品在批准後的安全性。該機構還可以 要求測試和監控計劃在產品商業化後對其進行監控,或者 施加其他條件,包括分銷限制或其他風險管理機制,包括REMS,以幫助確保產品的益處大於 潛在風險。REMS可以包括藥物指南、醫療保健專業人員的溝通計劃和確保安全使用的要素(ETASU)。ETASU可包括但不限於處方或配藥的特殊培訓或認證、僅在某些情況下的配藥、特殊監測和專利登記處的使用。FDA可以根據上市後研究或監督計劃的結果阻止或限制產品的進一步銷售。 批准後,批准的產品的許多類型的更改,如增加新的 適應症、製造更改和額外的標籤聲明,要接受進一步的 檢測要求和食品及藥物管理局的審查和approval. ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查聲明 財務非財務 信息 Argenx 2023年年度報告美國生物製品許可和監管|71 |
| Such regulatory reviews can result in denial or modification of the planned changes, or requirements to conduct additional tests or evaluations that can substantially delay or increase the cost of the planned changes. Fast Track, Breakthrough Therapy and Priority Review Designations The FDA is authorized to designate certain products for expedited review if they are intended to address an unmet medical need in the treatment of a serious or life-threatening disease or condition. These programs are referred to as fast track designation, breakthrough therapy designation and priority review designation. The FDA may designate a product for fast track review if it is intended, whether alone or in combination with one or more other products, for the treatment of a serious or life-threatening disease or condition, and it demonstrates the potential to address unmet medical needs for such a disease or condition. For fast track products, sponsors may have greater interactions with the FDA and the FDA may initiate review of sections of a fast track product’s application before the application is complete. This rolling review may be available if the FDA determines, after preliminary evaluation of clinical data submitted by the sponsor, that a fast track product may be effective. The sponsor must also provide, and the FDA must approve, a schedule for the submission of the remaining information and the sponsor must pay applicable user fees. However, the FDA’s goal for reviewing a rolling review does not begin until the last section of the application is submitted. Fast track designation may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process. A product may be designated as a breakthrough therapy if it is intended, either alone or in combination with one or more other products, to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. The FDA may take certain actions with respect to breakthrough therapies, including holding meetings with the sponsor throughout the development process; providing timely advice to the product sponsor regarding development and approval; involving more senior staff in the review process; assigning a cross-disciplinary project lead for the review team; and taking other steps to design the clinical trials in an efficient manner. The FDA may designate a product for priority review if it is a product that treats a serious condition and, if approved, would provide a significant improvement in safety or effectiveness. The FDA determines, on a case-by-case basis, whether the proposed product represents a significant improvement when compared with other available therapies. Significant improvement may be illustrated by evidence of increased effectiveness in the treatment of a condition, elimination or substantial reduction of a treatment-limiting product reaction, documented enhancement of patient compliance that may lead to improvement in serious outcomes and evidence of safety and effectiveness in a new subpopulation. A priority designation is intended to direct overall attention and resources to the evaluation of such applications, and to shorten the FDA’s goal for taking action on a marketing application from 10 months to 6 months. Accelerated Approval Pathway The FDA may grant accelerated approval to a product for a serious or life-threatening condition that provides meaningful therapeutic advantage to patients over existing treatments based upon a determination that the product has an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit or on a clinical endpoint that can be measured earlier than an effect on irreversible morbidity or mortality (IMM) and that is reasonably likely to predict an effect on IMM or other clinical benefit, taking into account the severity, rarity, or ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 72 |
| 疾病的流行情況和可用或缺乏替代治療。獲得加速批准的產品 必須滿足與獲得傳統批准的產品在安全性和有效性方面相同的法定標準。 就加速批准而言,代理終點是一個標記,例如 實驗室測量、放射圖像、體徵或其他被認為可預測臨牀益處的指標,但其本身並不是臨牀效益的衡量標準。代理終端通常比臨牀終端更容易或更快速地進行測量。中間臨牀終點是對治療效果的測量,被認為合理地可能預測產品的臨牀益處,例如對IMM的影響。FDA在基於中間臨牀終點的加速審批方面的經驗有限,但已 表示,此類終點通常可以支持加速審批,如果終點測量的治療效果本身不是臨牀益處和傳統審批的基礎,如果有依據得出治療效果合理地 可能預測產品的最終臨牀益處。 加速審批途徑最常用於以下情況: 病程較長,需要延長時間來衡量產品的預期臨牀 效益,即使對代孕藥或中間臨牀終點的影響迅速發生 。因此,加速審批被廣泛用於開發和批准用於治療各種癌症的產品,這些癌症的治療目標通常是提高存活率或降低發病率,而典型疾病的持續時間 病程需要漫長的、有時甚至是大型的臨牀試驗來證明臨牀或存活的好處。 加速批准的途徑通常取決於贊助商同意以勤奮的方式進行批准後的驗證性臨牀試驗或研究,以驗證和 描述產品的臨牀益處。因此,在此基礎上批准的候選產品 必須遵守嚴格的上市後合規性要求,包括完成 階段4或批准後臨牀試驗,以確認對臨牀終點的影響。這些 驗證性臨牀試驗必須經過盡職調查才能完成,FDA可能要求 在批准之前設計、啟動和/或完全納入驗證性臨牀試驗。如果在上市後研究期間未能進行所需的批准後研究或確認臨牀益處,將允許FDA加速將該產品從市場上召回 。除非FDA另行通知,否則根據加速法規批准的所有候選產品的促銷材料都必須經過 機構的事先審查。2022年12月頒佈的《食品和藥物綜合改革法案》(FDORA) 包括了與加速審批途徑有關的條款。根據FDORA,FDA 被授權要求在批准之前或在批准後的指定時間段內進行批准後臨牀試驗。FDORA還要求FDA規定任何所需的批准後臨牀試驗的條件不遲於批准後180天,並且不少於此後每180天一次,直到此類臨牀試驗完成或終止。此類條件可能包括設定里程碑,如臨牀試驗完成的目標日期或要求贊助商提交進度報告。 FDORA還允許FDA對 未能盡職進行所需的批准後臨牀試驗,包括未能滿足FDA指定的任何要求條件或未能及時提交報告,採取執法行動或提起刑事訴訟。 美國的孤兒藥物指定 旨在鼓勵贊助商開發針對罕見疾病或疾病的產品 。在美國,罕見疾病或疾病在法律上被定義為在美國影響不到200,000人或在美國影響超過200,000人並且沒有合理的expectation ar Gr g oup enx Factors Risk Go Corporate vernance資本的疾病 股票財務 審查報表 財務非財務 信息 Argenx年度報告2023年美國生物製品許可和監管|73 |
| that the cost of developing and making available the product for the disease or condition will be recovered from sales of the product in the U.S. Orphan drug designation qualifies a company for tax credits and market exclusivity for seven years following the date of the product’s marketing approval if granted by the FDA and if it is the first FDA approval for that product for the disease for which it has such designation. An application for designation as an orphan product can be made any time prior to the filing of an application for approval to market the product. If the FDA grants orphan drug designation, the generic identity of the product and its potential orphan use are disclosed publicly by the FDA. The product must then go through the review and approval process like any other product in order to be marketed. A sponsor may request orphan drug designation of a previously unapproved product or new orphan indication for an already marketed product. In addition, a sponsor of a product that is otherwise the same product as an already approved orphan drug may seek and obtain orphan drug designation for the subsequent product for the same rare disease or condition if it can present a plausible hypothesis that its product may be clinically superior to the first drug. Whether a large molecule product (i.e., a biological product) is the same as another product is based on whether the two products have the same principal molecular structural features. More than one sponsor may receive orphan drug designation for the same product for the same rare disease or condition, but each sponsor seeking orphan drug designation must file a complete request for designation. If orphan drug exclusivity is granted by the FDA, the period of exclusivity begins on the date that the marketing application is approved by the FDA and applies only to the indication for which the product has been designated. The FDA may approve a second application for the same product for a different use or a second application for a clinically superior version of the product for the same use. The FDA cannot, however, approve the same product made by another sponsor for the same indication during the market exclusivity period unless it has the consent of the sponsor or the sponsor is unable to provide sufficient quantities of the product. Post-Approval Regulation If regulatory approval for marketing of a product or new indication for an existing product is obtained, the sponsor will be required to comply with all post-approval regulatory requirements as well as any post-approval requirements that the FDA has imposed as part of the approval process. The sponsor will be required to report certain adverse reactions and production problems to the FDA, provide updated safety and efficacy information and comply with requirements concerning advertising and promotional labeling. Manufacturers and other parties involved in the drug supply chain for prescription drug and biological products must also comply with product tracking and tracing requirements and for notifying the FDA of counterfeit, diverted, stolen and intentionally adulterated products or products that are otherwise unfit for distribution in the U.S. Manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with ongoing regulatory requirements, including cGMPs, which impose certain procedural and documentation requirements upon manufacturers. Accordingly, the sponsor and its third-party manufacturers must continue to expend time, money and effort in the areas of production and quality control to maintain compliance with cGMPs and other regulatory requirements. A biological product may also be subject to official lot release, meaning that the manufacturer is required to perform certain tests on each lot of the product before it is released for distribution. If the product is subject to official lot release, the manufacturer must submit samples of each lot, together with a release protocol showing a summary of the history of ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 74 |
| 批次的製造和製造商對批次進行的所有測試的結果, 提交給FDA。此外,FDA可能還會對一些 產品的批次進行某些驗證性測試,然後再放行批次進行分銷。最後,FDA將在實驗室進行與藥品的安全性、純度、效力和有效性有關的研究。 任何處方藥生物製品和藥品樣本的分銷都必須符合美國處方藥營銷法和PHSA。 一旦獲得批准,如果 不符合監管要求和標準,或者產品上市後出現問題,FDA可以撤銷或暫停對BLA的批准。產品後來發現以前未知的問題 ,包括意外嚴重性或頻率的不良事件,或製造工藝,或未能遵守法規要求,可能導致 修訂批准的標籤以添加新的安全信息;實施上市後 研究或臨牀試驗以評估新的安全風險;或根據REMS計劃實施分銷或其他 限制。FDA還有權要求在某些情況下進行產品有效性降低的上市後研究,並可能要求對與新的有效性降低信息相關的更改進行標籤。未能保持監管合規性的其他潛在後果包括: ·對產品營銷或製造的限制,產品從市場上完全撤回或產品召回; ·罰款、無標題信件或警告信或批准後臨牀試驗的擱置; ·FDA拒絕批准未決申請或已批准申請的補充申請,或暫停或吊銷產品許可證批准; ·產品扣押或扣留,或拒絕允許產品進出口;或 ·禁令或施加民事或刑事處罰。 FDA嚴格監管上市產品的營銷、標籤、廣告和促銷。藥品只能按照經批准的適應症和經批准的標籤的規定進行促銷。儘管 醫生可能會將合法提供的產品開給未經批准的用途或在患者 中未在產品批准的標籤中描述的人羣中使用(稱為“標籤外使用”),但擁有批准產品的公司不得營銷或推廣此類標籤外用途。FDA不規範醫生在選擇治療時的行為,但FDA法規確實對製造商關於非標籤使用的溝通施加了嚴格的限制。FDA和其他機構積極執行禁止推廣標籤外用途的法律和法規,被發現不當推廣標籤外用途的公司可能會承擔重大責任,包括聯邦和州當局的調查。處方藥生物製品宣傳材料必須在首次使用或首次發表時提交給FDA。 根據2003年修訂的《兒科研究公平法》(修訂,PREA),兒科研究和排他性 ,其BLA或補充劑 必須包含足夠的數據,以評估 產品在所有相關兒科人羣中聲稱的適應症的安全性和有效性,並支持該產品對每個兒科人羣安全有效的 劑量和給藥。申辦方還必須在評估數據之前提交兒科臨牀試驗計劃。 這些計劃必須包含申請人計劃進行的擬議兒科臨牀試驗或研究的大綱,包括臨牀試驗目標和設計、任何延期或豁免請求以及法規要求的其他信息。然後,申請人、FDA和FDA的內部審查委員會必須審查提交的信息,相互協商,並就最終計劃達成一致。食品和藥物管理局或申請人可以在任何time. ar Gr g oup enx Factors Risk Go Corporate vernance資本公司請求修改該計劃 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告美國生物製品許可和監管|75 |
| The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of some or all pediatric data until after approval of the product for use in adults, or full or partial waivers from the pediatric data requirements. Unless otherwise required by regulation, PREA does not apply to a biologic for an indication for which orphan designation has been granted, except that PREA will apply to an original BLA for a new active ingredient that is orphan-designated if the biologic is a molecularly targeted cancer product intended for the treatment of an adult cancer and is directed at a molecular target that FDA determines to be substantially relevant to the growth or progression of a pediatric cancer. Pediatric exclusivity is another type of non-patent marketing exclusivity in the U.S. and, if granted, provides for the attachment of an additional six months of marketing protection to the term of any existing regulatory exclusivity. This six-month exclusivity may be granted if a BLA sponsor submits pediatric data that fairly respond to a written request from the FDA for such data. The data do not need to show the product to be effective in the pediatric population studied; rather, if the clinical trial is deemed to fairly respond to the FDA’s request, the additional protection is granted. If reports of requested pediatric studies are submitted to and accepted by the FDA within the statutory time limits, whatever statutory or regulatory periods of exclusivity cover the product are extended by six months. Biosimilars and Exclusivity The Biologics Price Competition and Innovation Act (BPCIA) established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable biosimilars. Under the BPCIA, an applicant may submit an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with” a previously approved biological product or “reference product.” For the FDA to approve a biosimilar product, it must find that there are no clinically meaningful differences between the reference product and proposed biosimilar product in terms of safety, purity and potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must find that the biosimilar product can be expected to produce the same clinical results as the reference product, and (for products administered multiple times) that the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved. Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version of that product if the FDA approves a full BLA for such product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. However, to rely on such exclusivities for establishing or protecting our market position is not without risk, as such laws are subject to changes by the legislature. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products. Products deemed interchangeable by the FDA may be substituted by pharmacies as dictated by individual state law. U.S. Patent Term Restoration Depending upon the timing, duration and specifics of FDA approval of our product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Act that permits restoration of the patent term of up to five years as compensation for patent term lost during the FDA regulatory review process. Patent-term restoration, however, cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date and only those claims covering such approved product, a method for ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Licensure and Regulation of Biologics in the U.S. | 76 |
| using it or a method for manufacturing it may be extended. The patent-term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application, except that the review period is reduced by any time during which the applicant failed to exercise due diligence. Only one patent applicable to an approved biologic is eligible for the extension and the application for the extension must be submitted within sixty (60) days of approval from FDA and prior to the expiration of the patent. The U.S. Patent and Trademark Office (USPTO), in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may apply for restoration of patent term for our currently owned or licensed patents to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA. Regulation and Procedures Governing Approval of Medicinal Products in the European Union and the United Kingdom In order to market any medicinal product outside of the U.S., a company also must comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of products. Whether or not it obtains FDA approval for a product, an applicant will need to obtain the necessary approvals by the comparable regulatory authorities before it can initiate clinical trials or marketing of the product in those countries or jurisdictions. Specifically, with respect to the EU, no medicinal product may be placed on the market of an EU Member State unless a marketing authorization has been issued by the competent authorities of that member state in accordance with Directive 2001/83/EC or a centralized marketing authorization has been granted in accordance with Regulation (EC) No 726/2004, read in conjunction with Regulation (EC) No 1901/2006 and Regulation (EC) No 1394/2007. Similar requirements apply in Great Britain. The process governing approval of medicinal products in the EU and Great Britain generally follows the same lines as in the U.S. It entails satisfactory completion of pharmaceutical development, pre-clinical trials and adequate and well-controlled clinical trials to establish the safety and efficacy of the medicinal product for each proposed indication. The EU also requires the submission to relevant competent authorities for clinical trials authorization and to the European Medicines Agency (EMA) or to competent authorities in EU Member States of a marketing authorization application (MAA) and granting of such MAA by these authorities before the product can be marketed and sold in the EU. Following the UK’s departure from the EU, a separate MAA is required in order to place medicinal products on the market in the Great Britain (England, Wales and Scotland) (under the Northern Ireland Protocol, the EU regulatory framework will continue to apply in Northern Ireland in this regard). Centralized EU marketing authorizations continue to be recognized, with new International Recognition Procedures anticipated. Clinical Trial Approval On January 31, 2022, the new Clinical Trials Regulation (EU) No 536/2014 (CTR) entered into application, and replaced the Clinical Trials Directive 2001/20/EC. The transitional provisions of the new CTR offered sponsors the possibility to choose between the requirements of the previous Directive and the new CTR if the request for authorization of a clinical trial was submitted by January 30, 2023. If the sponsor chose to submit under the previous Directive, the clinical trial continues to be governed by the Directive until three years after the new CTR became applicable (i.e., January 30, 2025). If a clinical trial continues for more than three years after the Regulation became applicable, the new CTR will at that time begin to apply to the clinical trial (i.e., from January 31, 2025). The new Regulation, which is directly applicable in all 1.8.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation and Procedures Governing Approval of Medicinal Products in the EU and the UK | 77 |
| 歐盟成員國,旨在簡化歐盟臨牀試驗的審批。新的CTR的主要特點包括:通過臨牀試驗信息系統的單一入口點簡化了申請程序;為申請準備並提交了一套單一的文件,以及簡化了臨牀試驗贊助商的報告程序;以及統一的臨牀試驗申請評估程序,分為兩部分(第一部分包含科學和醫藥產品文件,第二部分包含國家和患者層面的文件)。第一部分由已提交臨牀試驗授權申請的所有歐盟成員國(涉及成員國)的所有歐盟成員國的主管當局進行協調審查進行評估。第二部分由每個有關成員國分別進行評估。對CTA的評估也設定了嚴格的截止日期。 在退出歐盟之前,英國通過2004年人用藥品(臨牀試驗)條例(修訂)將2001/20/EC指令實施為國家法律。 然而,新的歐盟CTR是在英國脱離歐盟後實施的,因此上一段中描述的新CTR不適用於英國。英國藥品監管機構MHRA就英國臨牀試驗立法的改革進行了諮詢,該立法於2022年3月結束。諮詢結果於2023年3月公佈,其中包括改革臨牀試驗立法框架的建議,儘管改革的內容和時間表尚未確定。因此,英國臨牀試驗的未來監管框架仍然不確定。 孤兒指定和排他性 條例(EC)第141/2000號和條例(EC)第847/2000號規定,產品可被歐盟委員會指定為孤兒藥物,前提是其贊助商能夠證明:(1)該產品旨在診斷、預防或治療危及生命或慢性衰弱的疾病,(2)(I)提出申請時,疾病在歐盟的患病率不超過萬分之五,或(Ii)在沒有激勵措施的情況下,產品在歐盟的銷售不太可能產生足夠的回報,以證明其開發所需的投資是合理的,以及(3)沒有令人滿意的方法來診斷、預防或治療已在歐盟授權的相關疾病,或者,如果存在此類方法,與適用於該疾病的產品相比,該產品必須具有顯著的好處。 指定孤兒可提供許多好處,包括費用減免和監管協助。如果授予孤兒藥品的營銷授權, 這將導致十年的市場獨佔期。在此市場排他期內,歐洲藥品管理局、歐盟委員會和歐盟成員國都不能接受申請,也不能批准“類似醫藥產品”的銷售授權。“類似的 藥品”被定義為含有與授權的孤兒藥品中含有的類似活性物質或 物質的藥品,其目的是用於 相同的治療適應症。但是,如果在第五年結束時確定該產品不再符合孤兒指定標準,則授權治療性 適應症的市場專營期可縮短至六年,因為例如, 該產品的利潤足夠高,不足以證明市場獨佔性是合理的。在非常特殊的情況下,市場排他性也可能被撤銷,例如:(I)確定類似的醫藥 產品更安全、更有效或在臨牀上更好;(Ii)授權孤兒產品的營銷授權持有人同意第二次孤兒申請;或 (Iii)授權孤兒產品的營銷授權持有人無法供應足夠的孤兒醫藥產品。在提交上市審批申請之前,必須要求指定為孤兒。指定孤兒不會在監管審批中傳達任何優勢,也不會縮短監管審批process. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 評審報表 財務非財務 信息 Argenx年度報告2023年歐盟和英國藥品審批條例和程序 |78 |
| Since January 1, 2021, a separate process for orphan designation has applied in Great Britain. There is now no pre-marketing authorization orphan designation (as there is in the EU) and the application for orphan designation will be reviewed by the MHRA, at the time of an MAA for a UK or Great Britain marketing authorization. The criteria are the same as in the EU, save that they apply to Great Britain only (e.g., there must be no satisfactory method of diagnosis, prevention or treatment of the condition concerned in Great Britain, as opposed to the EU, and the prevalence of the condition must be no more than five in 10,000 persons in Great Britain). Marketing Authorization To obtain a marketing authorization for a product under the EU regulatory system, an applicant must submit an MAA, either to the EMA using the centralized procedure or to competent authorities in the EU using the other procedures (decentralized procedure, national procedure, or mutual recognition procedure). A marketing authorization may be granted only to an applicant established in the EU. Regulation (EC) No. 1901/2006 provides that prior to obtaining a marketing authorization in the EU, an applicant must demonstrate compliance with all measures included in an EMA-approved pediatric investigation plan (PIP), covering all subsets of the pediatric population, unless the EMA has granted a product-specific waiver, class waiver, or a deferral for one or more of the measures included in the PIP. The centralized procedure provides for the grant of a single marketing authorization by the EU Commission that is valid for all EEA Member States. Pursuant to Regulation (EC) No. 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products (gene therapy, somatic cell therapy or tissue engineered products) and products with a new active substance indicated for the treatment of certain diseases, including products for the treatment of cancer and auto-immune diseases and other immune dysfunctions and neurodegenerative disorders. The centralized procedure is optional for products that contain a new active substance for any other indications, which are a significant therapeutic, scientific or technical innovation and whose authorization would be in the interest of public health in the EU. Under the centralized procedure, the EMA’s Committee for Medicinal Products for Human Use (CHMP) is responsible for conducting the assessment of a product to define its risk/benefit profile. The CHMP recommendation is then sent to the EU Commission, which adopts a decision binding in all EEA Member States. Under the centralized procedure, the maximum timeframe for the evaluation of an MAA is 210 days, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions asked by the CHMP. Clock stops may extend the timeframe of evaluation of an MAA considerably beyond 210 days. Accelerated evaluation may be granted by the CHMP in exceptional cases, when a medicinal product is of major interest from the point of view of public health and, in particular, from the viewpoint of therapeutic innovation. If the CHMP accepts such a request, the time limit of 210 days will be reduced to 150 days (excluding clock stops), but it is possible that the CHMP may revert to the standard time limit for the centralized procedure if it determines that it is no longer appropriate to conduct an accelerated assessment. Following the departure of the UK from the EU, Great Britain is no longer covered by centralized marketing authorizations (under the Northern Ireland Protocol, centralized EU authorizations will continue to be recognized in Northern Ireland). All medicinal products with a current centralized authorization were automatically converted to UK marketing authorizations on January 1, 2021, and there is a further period to December 31, 2023, during which the MHRA may rely on a decision taken by the EU Commission on the approval of a new marketing authorization in the centralized procedure, in order to more quickly grant a new ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation and Procedures Governing Approval of Medicinal Products in the EU and the UK | 79 |
| Great Britain marketing authorization European Commission Decision Reliance Procedure (ECDRP). A separate application is, however, still required. The December 31, 2023 date by which the ECDRP was due to draw to a close is currently subject to a public consultation. From January 1, 2024, a new International Recognition Procedure will become available, which is a new licensing route for medicines allowing the UK to recognize approvals from specified Reference Regulators, including the FDA and the EMA and EU member state competent authorities. European Data and Market Exclusivity In the EU, innovative medicinal products, approved on the basis of a complete independent data package, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. The data exclusivity, if granted, prevents generic or biosimilar applicants from referencing the innovator’s preclinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the EU, for a period of eight years from the date on which the reference product was first authorized in the EU. During the additional two-year period of market exclusivity, a generic or biosimilar MAA can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar product can be marketed in the EU until the expiration of the market exclusivity period. The overall ten-year period will be extended to a maximum of 11 years if, during the first 8 years of those 10 years, the marketing authorization holder obtains a marketing authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are determined to bring a significant clinical benefit in comparison with currently approved therapies. There is no guarantee that a product will be considered by the EMA to be an innovative medicinal product, and products may not qualify for data exclusivity. Even if a product is considered to be an innovative medicinal product so that the innovator gains the prescribed period of data exclusivity, another company nevertheless could also market another version of the product if such company obtained a marketing authorization based on an MAA with a complete independent data package of pharmaceutical tests, preclinical tests and clinical trials. Similar arrangements apply in the UK. Periods of Authorization and Renewals A marketing authorization is valid for five years, in principle, and it may be renewed after five years on the basis of a reevaluation of the risk benefit balance by the EMA for a centrally authorized product, or by the competent authority of the authorizing member state for a nationally authorized product. Once renewed, the marketing authorization is valid for an unlimited period, unless the EU Commission or the competent authority decides, on justified grounds relating to pharmacovigilance, to proceed with one additional five-year renewal period. Any marketing authorization that is not followed by the placement of the drug on the EU market (in the case of the centralized procedure) or on the market of the authorizing member state (for a nationally authorized product) within three years after authorization, or if the drug is removed from the market for three consecutive years, ceases to be valid. In Great Britain, centrally authorized products converted from EU to UK marketing authorizations will have the same renewal date. Regulatory Requirements after Marketing Authorization Following approval, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of the medicinal product. These include compliance with the EU’s stringent pharmacovigilance or safety reporting rules, pursuant to which post-authorization studies and additional monitoring obligations can be imposed. In addition, the manufacturing of authorized products, for which a separate manufacturer’s license is mandatory, must also be conducted in strict compliance with the EMA’s GMP requirements and comparable requirements of other regulatory bodies in ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation and Procedures Governing Approval of Medicinal Products in the EU and the UK | 80 |
| the EU, which mandate the methods, facilities and controls used in manufacturing, processing and packing of products to assure their safety and identity. Finally, the marketing and promotion of authorized products, including industry-sponsored continuing medical education and advertising directed toward the prescribers of products and/or the general public, are strictly regulated in the EU under Directive 2001/83/EC, as amended. The aforementioned EU rules are generally applicable in the EEA, and similar arrangements apply in the UK. Proposal for new EU Pharmaceutical Legislation On April 26, 2023, the European Commission has published a proposal for a new directive (COM/2023/192 final) and a new regulation (COM/2023/193 final), which would revise and replace the existing general pharmaceutical legislation, including e.g., Directive 2001/83/EC, as well as Regulations (EC) No. 726/2004, No. 141/2000, or No. 1901/2006 (EU Pharmaceutical Legislation). This proposal is currently undergoing the ordinary legislative procedure in the European Parliament and Council of the European Union and is therefore still subject to changes. If at all, the EU Pharmaceutical Legislation is expected to be implemented at the earliest in the next few years. Prevention and mitigation of medicine shortages, simplification of the market entry of generics and biosimilars, the reduction of the regulatory burden (e.g. by increased digitalization) and the implementation of a new regime for data and/or market exclusivity (e.g. by reducing the minimum period while introducing factors that, if met, prolong protections for marketing authorization holders) are among the major objectives pursued by the European Commission. Brexit and the Regulatory Framework in the UK On January 31, 2020, the UK officially withdrew from the EU (Brexit). Provisionally since January 1, 2021 and formally since May 1, 2021, the EU and UK’s trade and cooperation agreement (TCA) includes specific provisions concerning pharmaceuticals, which include the mutual recognition of GMP, inspections of manufacturing facilities for medicinal products and GMP documents issued, but does not foresee wholesale mutual recognition of UK and EU pharmaceutical regulations. The UK has implemented EU legislation on the marketing, promotion and sale of medicinal products through the Human Medicines Regulations 2012 (as amended) and has not yet enacted significant legislative change in this area following its exit from the EU. The regulatory regime in Great Britain therefore largely aligns with current EU regulations. However, these regimes may diverge increasingly as time passes, now that Great Britain’s regulatory system is independent from the EU, and the TCA does not provide for mutual recognition of UK and EU pharmaceutical legislation. For example, as already explained, the new Clinical Trials Regulation which became effective in the EU on January 31, 2022 has not been implemented into UK law, and a separate application will need to be submitted for clinical trial authorization in the UK. Furthermore, the position in Northern Ireland differs in certain respects from that of the rest of the UK (England, Wales and Scotland) as some EU rules continue to be applicable to Northern Ireland following the UK’s departure from the EU. Regulation and Procedures Governing Approval of Medicinal Products in Japan In order to market any medical products in Japan, a company must comply with numerous and varying regulatory requirements in Japan regarding quality, safety and efficacy in the context, among other things, of clinical trials, marketing approval, commercial sales and distribution of products. A person who manufactures or markets medical products in Japan is subject to the supervision of the Ministry of Health, Labour and Welfare (MHLW), primarily under the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices 1.8.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation and Procedures Governing Approval of Medicinal Products in Japan | 81 |
| (Pharmaceutical and Medical Devices Act). This entails the satisfactory completion of pharmaceutical development, preclinical studies and adequate and well-controlled clinical trials to establish the safety and efficacy of the medical product for each proposed indication. It also requires the filing of a notification of clinical trials with the Pharmaceuticals and Medical Devices Agency (Japan) (PMDA) and the obtaining of marketing approval from the relevant authorities before the product can be marketed and sold in the Japanese market. Business License Under the Pharmaceutical and Medical Devices Act, a person is required to obtain from the MHLW a marketing license in order to conduct the business of marketing, leasing or providing medical products that are manufactured (or outsourced to a third party for manufacturing) or imported by such person. Also, in order to conduct the business of manufacturing medical products which will be marketed in Japan, a person is required to obtain from the MHLW a manufacturing license for each manufacturing site. Marketing Approval Under the Pharmaceutical and Medical Devices Act, it is generally required to obtain marketing approval from the MHLW for the marketing of each medical product. An application for marketing approval must be made through the PMDA, which implements a marketing approval review. Clinical Trial Under the Pharmaceutical and Medical Devices Act, it is required to file notification of clinical trials with the PMDA. Also, the data of clinical trials and other pertinent data, which must be attached for an application for marketing approval, must be obtained in compliance with the standards established by the MHLW, such as GLPs and GCPs stipulated by the ministerial ordinances of the MHLW. Regulatory Requirements after Marketing Approval A marketing license-holder that has obtained marketing approval for a new pharmaceutical must have that pharmaceutical re-examined by the PMDA for a specified period after receiving marketing approval. Such re-examination period for VYVGART is stated to be 10 years after the marketing approval in January 2022. The purpose of this re-examination process is to ensure the safety and efficacy of a newly approved pharmaceutical by imposing on the marketing license-holder the obligation to gather clinical data for a certain period after the marketing approval was granted so that the PMDA has the opportunity to re-examine the product. Results of usage and other pertinent data must be attached for an application for a re-examination. A marketing license holder that has obtained a marketing approval is also required to investigate, among other things, the results of usage and to periodically report to the PMDA pursuant to the Pharmaceutical and Medical Devices Act. Price Regulation In Japan, public medical insurance systems cover virtually the entire Japanese population. The public medical insurance system, however, does not cover any medical product which is not listed on the National Health Insurance (NHI) price list published by the Minister of the MHLW. Accordingly, a marketing license-holder of medical products must first have a new medical product listed on the NHI price list in order to obtain its coverage under the public medical insurance system. The NHI price list listed VYVGART in April 2022. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulation and Procedures Governing Approval of Medicinal Products in Japan | 82 |
| The NHI price of a medical product is determined either by price comparison of comparable medical products with necessary adjustments for innovativeness, usefulness or size of the market; or, in the absence of comparable medical products, by the cost calculation method, determined after considering of the opinion of the manufacturer. Prices on the NHI price list will be subject to revision, generally once every year, on the basis of the actual prices at which the medical products are purchased by medical institutions. Coverage, Pricing and Reimbursement Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we may obtain regulatory approval. Even if our product candidates are approved for marketing, sales of such product candidates will depend, in part, on the extent to which third-party payors, including government health programs in the U.S. (such as Medicare and Medicaid), commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such product candidates. Moreover, increasing efforts by governmental and third-party payors in the EU, the U.S. and other markets to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. We expect to experience pricing pressures in connection with the sale of any of our product candidates due to the trend toward managed healthcare, the increasing influence of health maintenance organizations and additional legislative changes. The downward pressure on healthcare costs in general, particularly prescription drugs and surgical procedures and other treatments, has become very intense. As a result, increasingly high barriers are being erected to the entry of new products. In the U.S. and markets in other countries, patients generally rely on third-party payors to reimburse all or part of the costs associated with their treatment. Adequate coverage and reimbursement from governmental healthcare programs, such as Medicare and Medicaid, and commercial payors is critical to new product acceptance. Patients are unlikely to use any product candidates we may develop unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of such product candidates. Factors payors consider in determining reimbursement are based on whether the product is (i) a covered benefit under its health plan; (ii) safe, effective and medically necessary; (iii) appropriate for the specific patient; (iv) cost-effective; and (v) neither experimental nor investigational. The Medicare and Medicaid programs increasingly are used as models for how private payors and other governmental payors develop their coverage and reimbursement policies for drugs and biologics. Some third-party payors may require pre-approval of coverage for new or innovative devices or drug therapies before they will reimburse healthcare providers who use such therapies. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement for our product candidates. No uniform policy for coverage and reimbursement for drug products exists among third-party payors in the U.S. Therefore, coverage and reimbursement for drug products can differ significantly from payor to payor. As a result, the coverage determination process is often a time-consuming and costly process that will require us to provide scientific and clinical support for the use of our products to each payor separately, with no assurance that coverage and adequate reimbursement will be applied consistently or obtained in the first instance. Each plan determines whether or not it will provide coverage for a product, what amount it will pay the manufacturer for the product, on what tier of its formulary the product will be placed and whether to require step therapy. The position of a product on a formulary generally determines the co-payment that a patient will need to make to obtain the product and can 1.8.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Coverage, Pricing and Reimbursement | 83 |
| strongly influence the adoption of a product by patients and physicians. Third-party payors may limit coverage to specific products on a formulary, which might not include all of the approved products for a particular indication. Additionally, a payor’s decision to provide coverage for a product does not imply that an adequate reimbursement rate will be approved. For example, the payor may require co-payments that patients find unacceptably high. Further, one payor’s determination to provide coverage for a product does not assure that other payors will also provide coverage and reimbursement for the product, and the level of coverage and reimbursement can differ significantly from payor to payor. Third-party payors are increasingly challenging the price and examining the medical necessity and cost-effectiveness of medical products and services and imposing controls to manage costs, especially drugs when an equivalent generic drug or a less expensive therapy is available. It is possible that a third-party payor may consider our product candidate and other therapies as substitutable and only offer to reimburse patients for the less expensive product. Even if we show improved efficacy or improved convenience of administration with our product candidate, pricing of existing drugs may limit the amount we will be able to charge for our product candidate. These payors may deny or revoke the reimbursement status of a given drug product or establish prices for new or existing marketed products at levels that are too low to enable us to realize an appropriate return on our investment in product development. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our product candidates and may not be able to obtain a satisfactory financial return on products that we may develop. Third-party payors may limit coverage to specific products on an approved list, also known as a formulary, which might not include all of the approved products for a particular indication. In Mainland China, the newly created National Healthcare Security Administration (NHSA) an agency responsible for administering Mainland China’s social security system, organized a price negotiation with drug companies for certain new drugs that had not been included in the NRDL at the time of the negotiation in November 2019, which resulted in an average price reduction by over 60% for 70 of the 119 drugs that passed the negotiation. NHSA, together with other government authorities, review the inclusion or removal of drugs from Mainland China’s National Drug Catalog for Basic Medical Insurance, Work-related Injury Insurance and Maternity Insurance, or provincial or local medical insurance catalogues for the national medical insurance program regularly, and the tier under which a drug or device will be classified, both of which affect the amounts reimbursable to program participants for their purchases of those drugs. These determinations are made based on a number of factors, including price and efficacy. We may also be invited to attend the price negotiation with NHSA upon receiving regulatory approval in Mainland China, but we will likely need to significantly reduce our prices, and to negotiate with each of the provincial healthcare security administrations on reimbursement ratios. On the other hand, if the NHSA or any of its local counterpart includes our drugs and devices in the national RDL or provincial RDL, which may increase the demand for our drug candidates and devices, our potential revenue from the sales of our drug candidates and devices may still decrease as a result of lower prices. Moreover, eligibility for reimbursement in Mainland China does not imply that any drug or device will be paid for in all cases or at a rate that covers our costs, including licensing fees, research, development, manufacture, sale and distribution. Furthermore, rules and regulations regarding reimbursement change frequently, in some cases at short notice, and we believe that changes in these rules and regulations are likely. Outside the U.S., we will face challenges in ensuring obtaining adequate coverage and payment for any product candidates we may develop. Pricing of prescription pharmaceuticals is subject to governmental control in many countries. In order to secure coverage and reimbursement for any product that might be approved for sale, we have needed and may need to conduct expensive pharmacoeconomic studies in order to demonstrate the medical necessity and cost-effectiveness of the product, and the cost of these studies would be in ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Coverage, Pricing and Reimbursement | 84 |
| addition to the costs required to obtain FDA or other comparable marketing approvals. Conducting such studies could be expensive, involve additional risk and result in delays in our commercialization efforts. Even after pharmacogenomic studies are conducted, product candidates may not be considered medically necessary or cost-effective. A decision by a third-party payor not to cover any product candidates we may develop could reduce physician utilization of such product candidates once approved and have a material adverse effect on our sales, results of operations and financial condition. Third-party reimbursement and coverage may not be adequate to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development. The insurance coverage and reimbursement status of newly approved products for orphan diseases is particularly uncertain, and failure to obtain or maintain adequate coverage and reimbursement for any such product candidates could limit our ability to generate revenue. Further, due to the COVID-19 pandemic, millions of individuals have lost/will lose employer-based insurance coverage, which may adversely affect our ability to commercialize our products. As noted above, in the U.S., we plan to have various programs to help patients afford our products, including patient assistance programs and co-pay coupon programs for eligible patients. More specifically, patients can enroll into MY VYVGART PATH™, a patient support program that provides personalized support from a nurse case manager and committed support team. In addition to providing support on questions on the treatment and on navigating the insurance process, the program provides a VYVGART Co-pay Program to eligible patients, aids in referring patients to charitable foundations that may be able to help with out-of-pocket costs and informs patients of financial assistance programs that may be available. The containment of healthcare costs also has become a priority of U.S. federal, state and international governments and the prices of pharmaceuticals have been a focus in this effort. Governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the U.S. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from list prices and are challenging the prices charged for medical products. We cannot be sure that reimbursement will be available for any future product candidate that we commercialize and, if reimbursement is available, the level of reimbursement. In addition, many pharmaceutical manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price and best price. Penalties may apply in some cases when such metrics are not submitted accurately and timely. Further, these prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our potential revenue from the sale of any products for which we may obtain approval. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more of our products for which we or our collaborators receive marketing approval, less favorable coverage policies and reimbursement rates may be implemented in the future. Obtaining and maintaining reimbursement status is time-consuming and costly. In the EU, pricing and reimbursement schemes vary widely from country to country. Some countries provide that products may be marketed only after a reimbursement price has been agreed. Some countries may require the completion of additional studies that compare the cost-effectiveness of a particular product candidate to currently available therapies (so called health technology assessments) in order to obtain reimbursement or pricing approval. For example, the EU provides options for its Member States to restrict the range of products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. EU Member States may approve a specific price ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Coverage, Pricing and Reimbursement | 85 |
| for a product or may instead adopt a system of direct or indirect controls on the profitability of the company placing the product on the market. Other Member States allow companies to fix their own prices for products but monitor and control prescription volumes and issue guidance to physicians to limit prescriptions. Recently, many countries in the EU have increased the amount of discounts required on pharmaceuticals and these efforts could continue as countries attempt to manage healthcare expenditures, especially in light of the severe fiscal and debt crises experienced by many countries in the EU. The downward pressure on healthcare costs in general, particularly prescription products, has become intense. As a result, increasingly high barriers are being erected to the entry of new products. Political, economic and regulatory developments may further complicate pricing negotiations, and pricing negotiations may continue after reimbursement has been obtained. Reference pricing used by various EU Member States and parallel trade (arbitrage between low-priced and high-priced Member States) can further reduce prices. Special pricing and reimbursement rules may apply to orphan drugs. Inclusion of orphan drugs in reimbursement systems tend to focus on the medical usefulness, need, quality and economic benefits to patients and the healthcare system as for any drug. Acceptance of any medicinal product for reimbursement may come with cost, use and often volume restrictions, which again can vary by country. In addition, results-based rules of reimbursement may apply. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products, if approved in those countries. Historically, products launched in the EU do not follow price structures of the U.S. and generally prices tend to be significantly lower. Outside the U.S., international operations are generally subject to extensive governmental price controls and other market regulations, and we believe the increasing emphasis on cost-containment initiatives in Europe, Canada and other countries has and will continue to put pressure on the pricing and usage of our product candidates. In many countries, the prices of medical products are subject to varying price control mechanisms as part of national health systems. Other countries allow companies to fix their own prices for medical products but monitor and control company profits. Additional foreign price controls or other changes in pricing regulation could restrict the amount that we are able to charge for our product candidates. Accordingly, in markets outside the U.S., the reimbursement for our products may be reduced compared with the U.S. and may be insufficient to generate commercially reasonable revenue and profits. The delivery of healthcare in the EU, including the establishment and operation of health services and the pricing and reimbursement of medicines, is almost exclusively a matter for national, rather than EU, law and policy. National governments and health service providers have different priorities and approaches to the delivery of healthcare and the pricing and reimbursement of products in that context. In general, however, the healthcare budgetary constraints in most EU Member States have resulted in restrictions on the pricing and reimbursement of medicines by relevant health service providers. Coupled with ever-increasing EU and national regulatory burdens on those wishing to develop and market products, this could prevent or delay marketing approval of our product candidates, restrict or regulate post-approval activities and affect our ability to commercialize any products for which we obtain marketing approval. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Coverage, Pricing and Reimbursement | 86 |
| Government Pricing and Reimbursement Programs for Marketed Drugs in the U.S. Medicaid, the 340B Drug Pricing Program, and Medicare Federal law requires that a pharmaceutical manufacturer, as a condition of having its drug and biological products receive federal reimbursement under Medicaid and Medicare Part B, must pay rebates to state Medicaid programs for all units of its covered outpatient drugs dispensed to Medicaid beneficiaries and paid for by a state Medicaid program under either a fee-for-service arrangement or through a managed care organization. This federal requirement is effectuated through a Medicaid drug rebate agreement between the manufacturer and the Secretary of U.S. Department of Health and Human Services (HHS). The Centers for Medicare & Medicaid Services (CMS) administers the Medicaid drug rebate agreements, which provide, among other things, that the drug manufacturer will pay rebates to each state Medicaid agency on a quarterly basis and report certain price information on a monthly and quarterly basis. The rebates are based on prices reported to CMS by manufacturers for their covered outpatient drugs. For non-innovator products, generally generic drugs marketed under abbreviated NDAs, the rebate amount is 13% of the average manufacturer price (AMP) for the quarter. The AMP is the weighted average of prices paid to the manufacturer (1) directly by retail community pharmacies and (2) by wholesalers for drugs distributed to retail community pharmacies. For innovator products (i.e., drugs that are marketed under NDAs or BLAs), the rebate amount is the greater of 23.1% of the AMP for the quarter or the difference between such AMP and the best price for that same quarter. The best price is essentially the lowest price available to non-governmental entities after accounting for discounts and rebates. Innovator products may also be subject to an additional rebate that is based on the amount, if any, by which the product’s AMP for a given quarter exceeds the inflation-adjusted baseline AMP, which for most drugs is the AMP for the first full quarter after launch. Since 2017, non-innovator products are also subject to an additional rebate. To date, the rebate amount for a drug has been capped at 100% of the AMP; however, effective January 1, 2024, this cap will be eliminated, which means that a manufacturer could pay a total rebate amount on a unit of the drug that is greater than the average price the manufacturer receives for the drug. The terms of participation in the Medicaid drug rebate program impose an obligation to correct the prices reported in previous quarters, as may be necessary. Any such corrections could result in additional or lesser rebate liability, depending on the direction of the correction. In addition to retroactive rebates, if a manufacturer were found to have knowingly submitted false information to the government, federal law provides for civil monetary penalties for failing to provide required information, late submission of required information, and false information. A manufacturer must also participate in a federal program known as the 340B drug pricing program in order for federal funds to be available to pay for the manufacturer’s drug and biological products under Medicaid and Medicare Part B. Under this program, the participating manufacturer agrees to charge certain safety net healthcare providers no more than an established discounted price for its covered outpatient drugs. The formula for determining the discounted price is defined by statute and is based on the AMP and the unit rebate amount as calculated under the Medicaid drug rebate program, discussed above. Manufacturers are required to report pricing information to the Health Resources and Services Administration (HRSA) on a quarterly basis. HRSA has also issued regulations relating to the calculation of the ceiling price as well as imposition of civil monetary penalties for each instance of knowingly and intentionally overcharging a 340B covered entity. 1.8.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Government Pricing and Reimbursement Programs for Marketed Drugs in the U.S. | 87 |
| Federal law also requires that manufacturers report data on a quarterly basis to CMS regarding the pricing of drugs that are separately reimbursable under Medicare Part B. These are generally drugs and biologics, such as injectable products, that are administered incident to a physician service and are not generally self-administered. The pricing information submitted by manufacturers is the basis for reimbursement to physicians and suppliers for drugs covered under Medicare Part B. Under the Inflation Reduction Act (IRA), manufacturers are also required to provide quarterly rebates for certain single-source drugs and biologics (including biosimilars) covered under Medicare Part B with prices that increase faster than the rate of inflation. This requirement started on January 1, 2023 for drugs approved on or before December 1, 2020 and begins six quarters after a drug is first marketed for all other drugs. As with the Medicaid drug rebate program, federal law provides for civil monetary penalties for failing to provide required information, late submission of required information, and false information. Recently, the Infrastructure Investment and Jobs Act added a requirement, effective January 1, 2023, for manufacturers of certain single-source drugs (including biologics and biosimilars) separately paid for under Medicare Part B for at least 18 months and marketed in single-dose containers or packages (known as refundable single-dose containers or single-use package drugs) to provide annual refunds for any portions of the dispensed drug that are unused and discarded if those unused or discarded portions exceed an applicable percentage defined by statute or regulation. Manufacturers will be subject to periodic audits and those that fail to pay refunds for their refundable single-dose containers or single-use package drugs shall be subject to civil monetary penalties. Medicare Part D provides prescription drug benefits for seniors and people with disabilities. Medicare Part D enrollees once had a gap in their coverage (between the initial coverage limit and the point at which catastrophic coverage begins) where Medicare did not cover their prescription drug costs, known as the coverage gap. However, beginning in 2019, Medicare Part D enrollees paid 25% of brand drug costs after they reached the initial coverage limit – the same percentage they were responsible for before they reached that limit – thereby closing the coverage gap from the enrollee’s point of view. Most of the cost of closing the coverage gap is being borne by innovator companies and the government through subsidies. Each manufacturer of drugs approved under NDAs or BLAs is required to enter into a Medicare Part D coverage gap discount agreement and provide a 70% discount on those drugs dispensed to Medicare Part D enrollees in the coverage gap, in order for its drugs to be reimbursed by Medicare Part D. Beginning in 2025, the IRA eliminates the coverage gap under Medicare Part D by significantly lowering the enrollee maximum out-of-pocket cost and requiring manufacturers to subsidize, through a newly established manufacturer discount program, 10% of Part D enrollees’ prescription costs for brand drugs above a deductible and below the out-of-pocket maximum, and 20% once the out-of-pocket maximum has been reached. Although these discounts represent a lower percentage of enrollees’ costs than the current discounts required below the out-of-pocket maximum (that is, in the coverage gap phase of Part D coverage), the new manufacturer contribution required above the out-of-pocket maximum could be considerable for very high-cost patients and the total contributions by manufacturers to a Part D enrollee’s drug expenses may exceed those currently provided. The IRA also requires manufacturers to provide annual Medicare Part D rebates for single-source drugs and biological products with prices that increase faster than the rate of inflation. The IRA also allows HHS to directly negotiate the selling price of a statutorily specified number of drugs and biologics each year that CMS reimburses under Medicare Part B and Part D. Only high-expenditure single-source biologics that have been approved for at least 11 years (7 years for single-source drugs) can qualify for negotiation, with the negotiated price taking effect two years after the selection year. Negotiations for Medicare Part D products begin in 2024 with the negotiated price taking effect in 2026, and negotiations for Medicare Part B products begin in 2026 with the negotiated price taking effect in 2028. In August 2023, HHS ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Government Pricing and Reimbursement Programs for Marketed Drugs in the U.S. | 88 |
| announced the 10 Medicare Part D drugs and biologics that it selected for negotiations, and by October 1, 2023, each manufacturer of the selected drugs signed a manufacturer agreement to participate in the negotiations. HHS will announce the negotiated maximum fair price by September 1, 2024, and this price cap, which cannot exceed a statutory ceiling price will come into effect on January 1, 2026. U.S. Federal Contracting and Pricing Requirements Manufacturers are also required to make their covered drugs, which are generally drugs approved under NDAs or BLAs, available to authorized users of the Federal Supply Schedule (FSS) of the General Services Administration. The law also requires manufacturers to offer deeply discounted FSS contract pricing for purchases of their covered drugs by the Department of Veterans Affairs, the Department of Defense, the Coast Guard, and the Public Health Service (including the Indian Health Service) in order for federal funding to be available for reimbursement or purchase of the manufacturer’s drugs under certain federal programs. FSS pricing to those four federal agencies for covered drugs must be no more than the Federal Ceiling Price (FCP), which is at least 24% below the Non-Federal Average Manufacturer Price (Non-FAMP) for the prior year. The Non-FAMP is the average price for covered drugs sold to wholesalers or other middlemen, net of any price reductions. The accuracy of a manufacturer’s reported Non-FAMPs, FCPs, or FSS contract prices may be audited by the government. Among the remedies available to the government for inaccuracies is recoupment of any overcharges to the four specified federal agencies based on those inaccuracies. If a manufacturer were found to have knowingly reported false prices, in addition to other penalties available to the government, the law provides for significant civil monetary penalties per incorrect item. Finally, manufacturers are required to disclose in FSS contract proposals all commercial pricing that is equal to or less than the proposed FSS pricing, and subsequent to award of an FSS contract, manufacturers are required to monitor certain commercial price reductions and extend commensurate price reductions to the government, under the terms of the FSS contract Price Reductions Clause. Among the remedies available to the government for any failure to properly disclose commercial pricing and/or to extend FSS contract price reductions is recoupment of any FSS overcharges that may result from such omissions. Healthcare Law and Regulation Healthcare providers and third-party payors play a primary role in the recommendation and prescription of pharmaceutical products that are granted marketing approval. Our current and future arrangements with providers, researchers, consultants, third-party payors and customers are subject to broadly applicable federal and state fraud and abuse, anti-kickback, false claims, transparency and patient privacy laws and regulations and other healthcare laws and regulations that may constrain our business and/or financial arrangements. Restrictions under applicable federal and state healthcare laws and regulations include, without limitation, the following: · the U.S. federal Anti-Kickback Statute (AKS) prohibits, among other things, persons and entities from knowingly and willfully soliciting, receiving, offering, or paying remuneration, directly or indirectly, in cash or in kind, to induce or reward either the referral of an individual for, or the purchase, order or recommendation of, any good or service, for which payment may be made, in whole or in part, under a federal healthcare program such as Medicare and Medicaid. This statute has been interpreted to apply to arrangements between pharmaceutical manufacturers on the one hand and prescribers, purchasers and formulary managers on the other. A person or entity can be found guilty of violating the AKS without actual knowledge of the statute or specific intent to violate it. In addition, the government may assert that a claim including items or services resulting from a violation of 1.8.6 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Healthcare Law and Regulation | 89 |
| 根據聯邦虛假索賠法案或聯邦民事罰款法規,AKS構成虛假或欺詐性索賠。違反AKS的行為可能會受到重大的民事和刑事處罰,包括監禁、罰款、行政民事罰款和被排除在聯邦醫療保健計劃之外。2020年12月2日,監察長辦公室(OIG)公佈了對AKS的進一步修訂。 在最終規則下,OIG在AKS下增加了對臨牀醫生、提供者和其他人之間的某些 協調護理和基於價值的安排的安全港保護。 該規則於2021年1月19日生效。我們繼續評估 規則將對我們的業務產生什麼影響(如果有的話); ·美國聯邦虛假索賠和民事罰款法律,包括民事虛假索賠法案和聯邦民事罰款法律,除其他外,對故意向美國聯邦政府提交或導致向美國聯邦政府提交虛假或欺詐性付款或批准索賠的個人或實體施加刑事和民事處罰,包括通過民事舉報人或準訴訟,虛假的記錄或陳述對虛假的 或欺詐性的向聯邦政府支付或傳輸金錢的義務具有重要意義,或者 故意作出虛假陳述以避免、減少或隱瞞向美國聯邦政府支付 金錢的義務。此外,就《虛假申報法》而言,政府可斷言,包括因違反AKS而產生的物品和服務的索賠構成虛假或欺詐性索賠。根據《虛假索賠法》,製造商即使不直接向政府付款人提交索賠,也可能被追究責任。 如果製造商被認為“導致”提交虛假或欺詐性索賠,他們也會承擔責任。虛假索賠法案還允許充當“告密者”的個人代表聯邦政府提起訴訟,指控違反了虛假索賠法案,並 分享任何金錢追回。當實體被確定違反了聯邦民事虛假索賠法案時,政府可以對每個虛假索賠施加民事罰款和懲罰,外加三倍損害賠償,並將該實體排除在聯邦醫療保險、醫療補助和其他聯邦醫療保健計劃之外; ·1996年《美國聯邦健康保險攜帶和責任法案》(HIPAA)規定,除其他事項外,對故意和故意執行或試圖執行詐騙任何醫療福利計劃的計劃, 或通過虛假或欺詐性的藉口、陳述或承諾獲得任何醫療福利計劃所擁有或在其監管或控制下的任何 金錢或財產, 施加刑事和民事責任,而無論薪酬如何(例如,公共或私人)或故意和故意偽造 ,隱瞞或掩蓋與醫療保健事項有關的醫療福利、項目或服務的交付或支付的重大事實或作出重大虛假的陳述 ;與AKS類似,個人或實體不需要 實際瞭解法規或違反法規的具體意圖即可實施違規;經2009年《衞生信息技術促進經濟和臨牀健康法案》(HITECH)及其實施條例修訂,並經2013年《綜合規則》再次修訂的《衞生信息技術促進經濟和臨牀健康法案》(HIPAA),該規則規定了某些義務,包括強制性的合同條款,涉及在未經承保實體(受《HIPAA綜合規則》)最終授權的情況下保護個人可識別健康信息的隱私、安全和傳輸 ,即某些承保的健康計劃、醫療保健票據交換所和醫療保健提供者及其商業夥伴,為他們或代表他們執行某些服務的承保實體的 獨立承包商或代理,涉及使用或披露個人身份的健康信息 。HITECH還創建了新的民事罰款等級,修訂了HIPAA,使民事和刑事處罰直接適用於商業夥伴和可能的其他人,並賦予州總檢察長新的權力,可以在聯邦法院提起民事訴訟,要求 損害賠償或禁令,以執行聯邦HIPAA法律,並要求 律師費和與提起聯邦民事訴訟相關的費用; ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告醫療法律法規|90 |
| · the federal transparency requirements known as the federal Physician Payments Sunshine Act, under the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 (collectively, the ACA), which requires certain manufacturers of drugs, devices, biologics and medical supplies to report annually to CMS information related to payments and other transfers of value made by that entity to physicians (currently defined to include doctors, dentists, optometrists, podiatrists and chiropractors), certain non-physician providers such as physician assistants and nurse practitioners and teaching hospitals, as well as ownership and investment interests held by physicians and their immediate family members. Failure to submit required information may result in civil monetary penalties for all payments, transfers of value or ownership or investment interests that are not timely, accurately, and completely reported in an annual submission; · federal government price reporting laws, which require us to calculate and report complex pricing metrics in an accurate and timely manner to government programs; · federal consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm consumers; · analogous state laws and regulations, including: state anti-kickback and false claims laws, which may apply to our business practices, including, but not limited to, research, distribution, sales and marketing arrangements and claims involving healthcare items or services reimbursed by any third party payor, including commercial insurers; state laws that require pharmaceutical companies to comply with the pharmaceutical industry’s voluntary compliance guidelines and the relevant compliance guidance promulgated by the U.S. federal government, or otherwise restrict payments that may be made to healthcare providers and other potential referral sources; state and local laws that require the licensure of sales representatives; state laws that require drug manufacturers to report information related to payments and other transfers of value to physicians and other healthcare providers or marketing expenditures and pricing information; state laws governing the privacy and security of health information in certain circumstances, many of which differ from each other in significant ways and may not have the same effect; and state laws related to insurance fraud in the case of claims involving private insurers; and · EU, UK and other foreign law equivalents, including reporting requirements detailing interactions with and payments to healthcare providers and data privacy and security laws and regulations that may be more stringent than those in the U.S. Some state laws require pharmaceutical companies to comply with the April 2003 Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s Code on Interactions with Healthcare Professionals, in addition to requiring pharmaceutical manufacturers to report information related to payments to physicians and other healthcare providers or marketing expenditures. Several states also impose other marketing restrictions or require pharmaceutical companies to make marketing or price disclosures to the state and require the registration of pharmaceutical sales representatives. State and foreign laws, including for example the EU General Data Protection Regulation (GDPR), also govern the privacy and security of health information in some circumstances, many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts. There are ambiguities as to what is required to comply with these state requirements and if we fail to comply with an applicable state law requirement, we could be subject to penalties. We have and will continue to spend substantial time and money to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations. Recent healthcare reform legislation has strengthened these federal and state healthcare laws. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Healthcare Law and Regulation | 91 |
| Other laws that may affect our ability to operate include: · the anti-inducement law prohibits, among other things, the offering or giving of remuneration, which includes, without limitation, any transfer of items or services for free or for less than fair market value (with limited exceptions), to a Medicare or Medicaid beneficiary that the person know or should know is likely to influence the beneficiary’s selection of a particular supplier of items or services reimbursable by a federal or state governmental program; and · European and other foreign law equivalents of each of the laws, including reporting requirements detailing interactions with and payments to healthcare providers. In the U.S., to help patients afford our approved product, we may utilize programs to assist them, including patient assistance programs and co-pay coupon programs for eligible patients. Government enforcement agencies have shown increased interest in pharmaceutical companies’ product and patient assistance programs, including reimbursement support services, and a number of investigations into these programs have resulted in significant civil and criminal settlements. In addition, at least one insurer has directed its network pharmacies to no longer accept co-pay coupons for certain specialty drugs the insurer identified. Our co-pay coupon programs could become the target of similar insurer actions. In addition, in November 2013, the CMS issued guidance to the issuers of qualified health plans sold through the ACA’s marketplaces encouraging such plans to reject patient cost-sharing support from third parties and indicating that the CMS intends to monitor the provision of such support and may take regulatory action to limit it in the future. The CMS subsequently issued a rule requiring individual market qualified health plans to accept third-party premium and cost-sharing payments from certain government-related entities. In September 2014, the OIG of the HHS issued a Special Advisory Bulletin warning manufacturers that they may be subject to sanctions under the AKS and/or civil monetary penalty laws if they do not take appropriate steps to exclude Part D beneficiaries from using co-pay coupons. Accordingly, companies exclude these Part D beneficiaries from using co-pay coupons. It is possible that changes in insurer policies regarding co-pay coupons and/or the introduction and enactment of new legislation or regulatory action could restrict or otherwise negatively affect these patient support programs, which could result in fewer patients using affected products, and therefore could have a material adverse effect on our sales, business, and financial condition. Third-party patient assistance programs that receive financial support from companies have become the subject of enhanced government and regulatory scrutiny. The OIG has established guidelines that suggest that it is lawful for pharmaceutical manufacturers to make donations to charitable organizations who provide co-pay assistance to Medicare patients, provided that such organizations, among other things, are bona fide charities, are entirely independent of and not controlled by the manufacturer, provide aid to applicants on a first-come basis according to consistent financial criteria and do not link aid to use of a donor’s product. However, donations to patient assistance programs have received some negative publicity and have been the subject of multiple government enforcement actions, related to allegations regarding their use to promote branded pharmaceutical products over other less costly alternatives. Specifically, in recent years, there have been multiple settlements resulting out of government claims challenging the legality of their patient assistance programs under a variety of federal and state laws. It is possible that we may make grants to independent charitable foundations that help financially needy patients with their premium, co-pay, and co-insurance obligations. If we choose to do so, and if we or our vendors or donation recipients are deemed to fail to comply with relevant laws, regulations or evolving government guidance in the operation of these programs, we could be subject to damages, fines, penalties, or other criminal, civil, or administrative sanctions or enforcement actions. We cannot ensure that our compliance controls, policies, and procedures will be sufficient to protect against acts of our employees, business partners, or vendors that may violate the laws or regulations of the jurisdictions in which we operate. Regardless of whether we have complied with the law, a ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Healthcare Law and Regulation | 92 |
| government investigation could impact our business practices, harm our reputation, divert the attention of management, increase our expenses, and reduce the availability of foundation support for our patients who need assistance. On November 30, 2020, the HHS published a regulation removing safe harbor protection for price reductions from pharmaceutical manufacturers to plan sponsors under Part D, either directly or through pharmacy benefit managers (PBMs), unless the price reduction is required by law. The rule also creates a new safe harbor for price reductions reflected at the point-of-sale, as well as a safe harbor for certain fixed fee arrangements between PBMs and manufacturers. The IRA delayed implementation of this change and new safe harbors for point-of-sale reductions in price for prescription pharmaceutical products and PBM service fees until January 1, 2032. Further, on December 31, 2020, CMS published a new rule, effective January 1, 2023, requiring manufacturers to ensure the full value of co-pay assistance is passed on to the patient or these dollars will count toward the AMP and Best Price calculation of the drug. On May 21, 2021, PhRMA sued the HHS in the U.S. District Court for the District of Columbia, to stop the implementation of the rule claiming that the rule contradicts federal law surrounding Medicaid rebates, and on May 17, 2022, the court vacated the rule. Violations of these laws or any future enacted laws can subject us to criminal, civil and administrative sanctions including monetary penalties, damages, fines, disgorgement, individual imprisonment and exclusion from participation in government funded healthcare programs, such as Medicare and Medicaid, additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm, and we may be required to curtail or restructure our operations. Moreover, we expect that there will continue to be federal and state laws and regulations, proposed and implemented, that could impact our future operations and business. Because of the breadth of these laws and the narrowness of the statutory exceptions and safe harbors available, it is possible that some of our business activities could be subject to challenge under one or more of such laws. Ensuring that our internal operations and future business arrangements with third parties comply with applicable healthcare laws and regulations will involve substantial costs. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other governmental laws and regulations that may apply to us, we may be subject to significant penalties, including administrative, civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs, individual imprisonment, reputational harm, and the curtailment or restructuring of our operations, as well as additional reporting obligations and oversight if we become subject to a corporate integrity agreement or other agreement to resolve allegations of non-compliance with these laws. Further, defending against any such actions can be costly and time-consuming, and may require significant financial and personnel resources. Therefore, even if we are successful in defending against any such actions that may be brought against us, our business may be impaired. If any of the physicians or other providers or entities with whom we expect to do business are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs and imprisonment. If any of the above occur, our ability to operate our business and our results of operations could be adversely affected. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Healthcare Law and Regulation | 93 |
| Healthcare Reform In the U.S., the EU and other foreign jurisdictions, there have been a number of legislative and regulatory changes to the healthcare systems that could affect our future results of operations. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs and improve the quality of healthcare. For example, the ACA, effective since March 2010, is a sweeping law intended to broaden access to health insurance, reduce or constrain the growth of healthcare spending, enhance remedies against fraud and abuse, add new transparency requirements for the healthcare and health insurance industries, impose new taxes and fees on the health industry and impose additional health policy reforms. Healthcare reforms that have been adopted, and that may be adopted in the future, could result in further reductions in coverage and levels of reimbursement for pharmaceutical products, increases in rebates payable under U.S. government rebate programs and additional downward pressure on pharmaceutical product prices. On September 9, 2021, the Biden administration published a wide-ranging list of policy proposals, most of which would need to be carried out by Congress, to reduce drug prices and drug payment. The HHS plan includes, among other reform measures, proposals to lower prescription drug prices, including by allowing Medicare to negotiate prices and disincentivize price increases, and to support market changes that strengthen supply chains, promote biosimilars and generic drugs, and increase price transparency. As discussed above, these initiatives culminated in the enactment of the IRA in August 2022, which allows, among other things, HHS to directly negotiate the selling price of statutorily specified number of drugs and biologics each year that CMS reimburses under Medicare Part B and Part D. Only high-expenditure single-source biologics that have been approved for at least 11 years (7 years for single-source drugs) can qualify for negotiation, with the negotiated price taking effect two years after the selection year. The negotiated prices, which will first become effective in 2026, will be capped at a statutory ceiling price. Negotiations for Medicare Part D products begin in 2024 with the negotiated price taking effect in 2026, and negotiations for Medicare Part B products begin in 2026 with the negotiated price taking effect in 2028. In August 2023, HHS announced the 10 Medicare Part D drugs and biologics that it selected for negotiations, and by October 1, 2023, each manufacturer of the selected drugs signed a manufacturer agreement to participate in the negotiations. HHS will announce the negotiated maximum fair price by September 1, 2024, and this price cap, which cannot exceed a statutory ceiling price will come into effect on January 1, 2026. The IRA also penalizes drug manufacturers that increase prices of Medicare Part B and Part D drugs at a rate greater than the rate of inflation. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. Manufacturers that fail to comply with the IRA may be subject to various penalties, including civil monetary penalties. The IRA also extends enhanced subsidies for individuals purchasing health insurance coverage in ACA marketplaces through plan year 2025. These provisions will take began taking progressively starting in 2023, although they may be subject to legal challenges. For example, the provisions related to the negotiation of selling prices of high-expenditure single-source drugs and biologics have been challenged in multiple lawsuits. Thus, while it is unclear how the IRA will be implemented, it will likely have a significant impact on the pharmaceutical industry. We expect that additional U.S. federal healthcare reform measures will be adopted in the future, any of which could limit the amounts that the U.S. federal government will pay for healthcare products and services, which could result in reduced demand for our product candidates or additional pricing pressures. 1.8.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Healthcare Reform | 94 |
| 此外,還提出了立法和監管建議,以擴大批准後的要求,並限制藥品的銷售和促銷活動。我們 無法確定是否會頒佈額外的法律變更,或FDA 法規、指南或解釋是否會更改,或此類更改 可能會對我們候選產品的上市審批(如果有)產生什麼影響。此外,美國國會加強了對FDA審批程序的審查,這可能會顯著推遲或阻止 上市審批,並使我們受到更嚴格的產品標籤和上市後條件及其他要求的約束。 美國個別州也越來越積極地通過立法和實施旨在控制藥品和生物產品定價的法規,包括價格或患者報銷限制、折扣、對某些 產品准入的限制以及營銷成本披露和透明度措施,在某些情況下,旨在鼓勵從其他國家進口和大宗採購。法律 對第三方付款人支付金額的強制價格控制或其他限制 可能會損害我們的業務、運營結果、財務狀況和前景。此外, 地區醫療當局和個別醫院越來越多地使用招標程序來確定哪些藥品和供應商將包括在其處方藥和其他醫療保健計劃中。這可能會降低對我們產品的最終需求或給我們的產品定價帶來壓力,這可能會對我們的業務、運營結果、財務狀況和前景產生負面影響。 在歐盟,類似的政治、經濟和監管發展可能會影響我們將當前或任何未來產品進行盈利商業化的能力。除了繼續 價格壓力和成本控制措施外,歐盟(如歐盟藥品立法)或成員國層面的立法發展可能會導致顯著的額外要求或障礙,這可能會增加我們的運營成本。在歐盟提供醫療保健,包括醫療服務的建立和運營以及藥品的定價和報銷,幾乎完全是國家法律和政策的問題,而不是歐盟的法律和政策。在這種情況下,各國政府和衞生服務提供者在提供衞生保健以及產品定價和報銷方面有不同的優先事項和方法。然而,總的來説,大多數歐盟成員國的醫療保健預算限制導致相關醫療服務提供者對藥品的定價和報銷進行限制。再加上希望開發和營銷產品的歐盟和國家監管機構的負擔不斷增加,這可能會阻止或推遲我們的候選產品的上市審批,限制或規範審批後的活動,並 影響我們將獲得上市審批的任何產品商業化的能力。在國際市場上,報銷和醫療保健支付制度因國家/地區的不同而有很大差異,許多國家/地區對特定產品和治療設置了價格上限。 我們無法預測美國或國外未來的立法或行政行動可能導致的政府監管的可能性、性質或程度。如果我們或我們的 協作者緩慢或無法適應現有要求的變化或採用新的要求或政策,或者如果我們或我們的協作者無法保持 合規,我們的候選產品可能會失去可能已獲得的任何監管批准,並且我們可能無法實現或保持盈利。這將對我們的business. ar Gr g oup enx Factors Risk Go Corporate vernance資本產生不利的 影響 股票財務 審核報表 財務非財務 信息 Argenx年度報告2023年醫療改革|95 |
| Environmental Issues which may Influence the Use of our Material Fixed Assets Our primary research and development activities take place in our facilities in Zwijnaarde, Belgium. For these activities we require, and have obtained, the necessary environmental and biohazard permits from the responsible governments, required by us for the manner in which we use said facilities. Documents on display We are subject to the information reporting requirements of the U.S. Securities Exchange Act of 1934, as amended (Exchange Act) applicable to foreign private issuers. Accordingly, we are required to file reports and other information with the SEC. Those reports may be inspected without charge at the locations described below. As a foreign private issuer, we are exempt from the rules under the Exchange Act related to the furnishing and content of proxy statements, and our officers, directors and principal shareholders are exempt from the reporting and short-swing profit recovery provisions contained in section 16 of the Exchange Act. In addition, we are not required under the Exchange Act to file periodic reports and financial statements with the SEC as frequently or as promptly as U.S. companies whose securities are registered under the Exchange Act. Nevertheless, we will file with the SEC an Annual Report containing financial statements that have been examined and reported on, with an opinion expressed by an independent registered public accounting firm. We maintain a corporate website at www.argenx.com. We make available on our website, free of charge, our Annual Report and the text of our reports on Form 6-K, including any amendments to these reports, as well as certain other SEC filings, as soon as reasonably practicable after they are electronically filed with or furnished to the SEC. Information contained on, or that can be accessed through, our website does not constitute a part of this Annual Report. We have included our website address in this Annual Report solely as an inactive textual reference. The SEC maintains a website (www.sec.gov) that contains reports and other information regarding registrants, such as argenx SE, that file electronically with the SEC. With respect to references made in this Annual Report to any contract or other document of argenx SE, such references are not necessarily complete and you should refer to the exhibits attached or included elsewhere to this Annual Report for copies of the actual contract or document. 1.8.8 1.9 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Environmental Issues which may Influence the Use of our Material Fixed Assets | 96 |
| Risk Factors 2.1 Summary Risk Factors 98 2.2 Risk Factors Related to argenx’s Financial Position and Need for Additional Capital 100 2.3 Risk Factors Related to Commercialization of argenx’s Products and Product Candidates, Including for New Indications 102 2.4 Risk Factors Related to Other Government Regulations 111 2.5 Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates 118 2.6 Risk Factors Related to argenx’s Dependence on Third Parties 123 2.7 Risk Factors Related to argenx’s Business and Industry 126 2.8 Risk Factors Related to argenx’s Intellectual Property 129 2.9 Risk Factors Related to argenx’s Organization and Operations 135 2.10 Risk Factors Related to the ADSs 139 2.11 Risk Factors Related to being a Foreign Private Issuer or a Dutch Company 141 2 argenx Annual Report 2023 Risk Factors | 97 |
| 2風險因素 彙總風險因素 我們的業務面臨重大風險,包括下文所述風險等。您 應認真考慮本年度報告和我們提交給美國證券交易委員會的其他 文件中列出的所有信息,包括我們面臨的和我們所在行業面臨的以下風險因素。如果發生任何此類風險,我們的業務、財務狀況或運營結果可能會受到重大影響和不利影響。這些並不是Argenx面臨的唯一風險。 Argenx目前未知或目前認為不重要或不具體的其他風險和不確定性也可能損害其業務、運營結果和財務狀況 。本報告還包含涉及風險和不確定性的前瞻性陳述。由於某些因素,包括本年度報告和美國證券交易委員會提交的其他文件中描述的風險,我們的實際結果可能與這些前瞻性陳述中預期的結果大不相同。請參閲“關於前瞻性陳述的告誡 聲明”。 ·我們自成立以來已遭受重大損失,預計在可預見的未來也將蒙受損失。我們可能永遠不會實現或保持盈利。 ·我們可能需要籌集大量額外資金,而這些資金可能無法以可接受的條款或根本無法提供給我們。 ·我們的資產、收益和現金流以及我們現金和現金等價物的投資可能會受到風險的影響,這些風險可能會導致損失,並影響這些投資的流動性。 ·我們將面臨巨大的挑戰,以成功地將我們的產品和其他候選產品商業化。 ·我們的產品和候選產品的商業成功,包括在新的 適應症或給藥方法中,將取決於市場接受的程度。 ·我們在藥物發現和開發工作方面面臨着激烈的競爭。 ·制定和未來的立法可能會影響對我們產品的需求,這可能會影響我們的業務和未來的運營結果。 ·我們受政府定價法律、法規和執法的約束。這些法律影響 我們可能向政府收取的產品價格以及我們的客户可能從政府獲得的報銷。我們不遵守這些法律可能會 損害我們的結果、運營和/或財務狀況。 ·我們可能無法為我們的 產品和候選產品獲得或保持足夠的保險或報銷狀態。 ·如果我們未能獲得孤立藥物指定,或者我們沒有覆蓋我們的產品和候選產品的有效和可強制執行的 專利,並且無法獲得和/或維護我們的產品或候選產品的 孤立藥物排他性,我們的競爭對手可能 能夠銷售產品來治療同樣的疾病,我們的收入可能會減少。 ·我們受到醫療保健法律、法規和執法的約束。不遵守這些法律可能會損害我們的運營結果和財務狀況。 ·我們業務的方方面面,從臨牀前、臨牀試驗、市場營銷到商業化,都受到嚴格監管,相關監管機構的任何拖延都可能危及我們的開發和審批流程,或導致其他暫停、 拒絕或撤回審批。 ·我們受隱私法、法規和潛在執法的約束。我們未能遵守這些法律 可能會損害我們的業績、運營和/或財務狀況。 2.1 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 argenx 2023年年報摘要|98 |
| ·未能在其他適應症中成功識別、選擇和開發VYVGART,或 其他產品或候選產品可能會削弱我們的增長能力。 ·VYVGART已獲得VYVGART批准的國家/地區的監管批准,可用於治療GMG。我們的其他產品和候選產品-包括efgartigimod、Empasiprubart和ARGX-119的其他 適應症或使用方法-處於臨牀前或臨牀開發階段,或正在等待上市批准。 ·我們的臨牀試驗已經失敗,未來可能會失敗,即使成功,我們也可能無法獲得監管機構對我們的產品和候選產品的批准,或者可能推遲監管批准。 ·我們的產品和候選產品可能會有嚴重的不良、不良或 不可接受的副作用,甚至導致死亡,我們或其他人在獲得上市批准後,可能會發現VYVGART或我們的任何產品或候選產品 導致的不良或不可接受的副作用。 ·如果我們的目標患者人數少於預期,我們無法成功 在我們的臨牀試驗中招募和留住患者,或者在此過程中遇到重大延誤, 我們可能無法充分認識到任何產品或候選產品的商業潛力。 ·我們依賴,並預計將繼續依賴,委託第三方進行我們的一些研究活動和臨牀試驗,以及我們現有和未來的研究計劃、產品和候選產品的部分開發和商業化。如果我們與此類第三方的關係不成功,我們的業務可能會受到不利的 影響。 ·由於我們在製造過程中依賴第三方而造成的中斷可能會 延遲或中斷我們的業務、產品開發和商業化努力。 ·未能充分執行或保護我們在產品、候選產品和平臺技術中的知識產權,可能會對我們的能力產生不利影響,使我們的營銷產品和候選產品為患者帶來最大價值。 ·如果我們的商標和商號沒有得到充分保護,我們可能無法 在我們感興趣的市場中建立知名度。 ·我們可能會遇到有效管理我們的增長以及我們日益增長的發展、監管和銷售和營銷能力的困難,這可能會擾亂我們的 運營。 ·我們的美國存託憑證和普通股的價格可能會波動,可能會因為我們無法控制的因素而波動 。活躍的公開交易市場可能無法持續。 ·我們美國存託憑證的持有者不被視為我們普通股的持有者,可能會受到轉讓其美國存託憑證和撤回相關普通股的限制。 ·我們是一家荷蘭歐洲上市公司(Societas Europaea或SE)。 我們股東的權利可能不同於受美國司法管轄區法律管轄的公司的股東權利。 ·美國的索賠。可能不會對我們或我們的管理層和董事會成員執行民事責任。 ·作為外國私人發行人,我們不受美國證券法的某些規則的約束, 向美國證券交易委員會提交的信息比美國公司少。 ·我們可能會失去外國私人發行人身份,這將要求我們遵守交易所法案的國內報告制度,並導致我們產生鉅額法律、 會計和其他費用。 ·如果出於美國聯邦收入的考慮,我們被歸類為被動外國投資公司 税務目的,這可能會對某些美國holders. ar Gr g oup enx Factors Risk Go Corporate vernance資本造成不利的美國税收後果 股票財務 審核報表 財務非財務 信息 argenx 2023年年度報告摘要|99 |
| Risk Factors Related to argenx’s Financial Position and Need for Additional Capital We have incurred significant losses since our inception and expect to incur losses for the foreseeable future. We may never achieve or sustain profitability. Since our inception, we have incurred significant operating losses, totaling $2,405 million of cumulative losses. To date we have commercialized VYVGART for the treatment of gMG. We do not currently have any marketing approvals for any other product candidates or VYVGART in other indications. Our losses resulted principally from costs incurred in research and development, preclinical testing and clinical development of our research programs, and from general and administrative costs associated with commercial roll out and expansion. We intend to continue to conduct research and development, preclinical testing, clinical trials and regulatory compliance activities as well as the continued commercialization of VYVGART and other products candidates, for current and future indications, and we intend to continue our efforts to expand our sales, marketing and distribution infrastructure. These expenses, together with anticipated general and administrative expenses, may result in incurring further losses for the foreseeable future. We anticipate that our operating expenses will increase if and as we execute our strategic objectives and as we experience delays or encounter issues relating thereto, including failed clinical trials, ambiguous clinical trial results, safety issues or other regulatory challenges. Although we have generated net product sales of $1.2 billion from global product net sales in fiscal year 2023, we can provide no assurances that we will be able to achieve or sustain profitability based on sales in that indication alone or that we will be able to receive regulatory approval of and commercialize VYVGART and VYVGART SC in other indications or in other countries. To become and remain profitable, we must succeed in developing and commercializing products that generate significant revenue. This will require us to be successful in a range of challenging activities, including completing preclinical testing and clinical trials of our products and our product candidates, discovering and developing additional products and product candidates, including new indications, obtaining regulatory approval, establishing manufacturing and marketing capabilities, obtaining funding or reimbursement for our products, and ultimately selling. Those activities are the drivers of our current path to profitability, however, we may not succeed in some or even all of these activities, and even if we do, we may not generate revenue that is significant enough to achieve profitability. We may need to raise substantial additional funding which may not be available to us on acceptable terms or at all. We have significant positions of cash and cash equivalents of $2,049 million and current financial assets of $1,131 million as of December 31, 2023. Developing products and product candidates, including new indications, and conducting clinical trials is time-intensive, expensive and risky. Our future capital requirements will depend on many factors, including: (i) the success, cost and timing of our development activities, preclinical testing and clinical trials for our product and product candidates, (ii) the time and costs involved in obtaining regulatory approvals and any delays we may encounter, including as we seek regulatory approval in additional jurisdictions or other indications, (iii) commercialization, manufacturing, sales and marketing of products and product candidates, (iv) securing adequate and uninterrupted supply chains, (v) the costs involved in growing our organization to the size needed to allow for the research, development and potential commercialization of our 2.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Financial Position and Additional Capital | 100 |
| 產品或候選產品,(Vi)提交專利申請以及 維護和執行專利或針對第三方提出的索賠或侵權進行辯護所涉及的成本,(Vii)維護我們現有的合作協議和簽訂新的合作協議,以及(Viii)收入的金額(如果有),我們可以直接或以專利使用費的形式從未來銷售我們的產品或候選產品(如果獲得批准)中獲得收入。 為我們的運營提供資金,我們可能需要通過組合 公共或私募股權或債務融資或其他來源來籌集額外資本,其中可能包括與第三方的合作 。例如,我們在2023年7月完成了一項全球發行,通過以每美國存托股份490.00美元的價格出售1,917,715張美國存託憑證和以每股436.37歐元的價格出售663,918股普通股,我們籌集了13億美元的毛收入。我們是否有能力以可接受的條款或根本不受限制地籌集額外資金,將取決於財務、經濟和 市場狀況以及其他因素,而我們對這些因素可能沒有控制權或控制能力有限。如果我們 在需要時無法籌集額外資本,或者如果條款不能接受,我們的 業務戰略可能會受到影響,我們可能會被迫推遲、減少或終止我們的一個或多個研發計劃或我們的任何 產品或候選產品的商業化,包括新的適應症,或者無法擴大我們的 業務或以其他方式利用我們的商機,所有這些都可能對我們的業務、財務狀況和運營結果產生 實質性的不利影響。 我們的資產,收益和現金流以及我們現金和現金等價物的投資可能會受到風險的影響,這些風險可能會導致損失,並 影響這些投資的流動性。 截至2023年12月31日,我們的現金和現金等價物以及流動金融資產為32億美元,而截至2022年12月31日為22億美元。我們所有的可用現金、現金等價物和流動金融資產都投資於往來賬户、儲蓄賬户、定期賬户或高流動性貨幣市場基金。根據我們的現金投資政策,未來的任何投資可能 包括定期存款、公司債券、商業票據、存單、政府證券和貨幣市場基金。這些投資可能會受到一般信貸、流動性、市場、通貨膨脹、外國貨幣和利率風險的影響,我們可能會在這些投資的公允價值中實現損失,或者完全損失這些投資,這將對我們的財務狀況產生負面影響。與我們的現金流和投資組合相關的市場風險可能會對我們的運營結果、流動性和財務狀況產生不利影響。 由於我們的業務範圍是國際化的,我們的資產、收益和現金流受到幾種貨幣匯率變動的影響,特別是美元、歐元和日元之間的匯率變動。隨着我們的產品(無論是由我們或我們的業務合作伙伴或我們的合作伙伴商業化)在這些司法管轄區獲得 營銷批准,我們在美國以外的收入將會增加。如果美元對特定外幣走弱,我們的收入將增加,對淨收入產生積極影響,但我們的總體支出將增加,產生負面影響。相反,如果美元兑特定外幣走強,我們的收入將減少,對 淨收入產生負面影響,但我們的總體支出將減少,從而產生積極影響。外匯匯率的持續波動可能會影響我們的經營業績和financial condition. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告財務狀況和額外資本|101 |
| 與Argenx產品商業化相關的風險因素 和候選產品,包括 新適應症 我們在產品和其他候選產品成功商業化後將面臨巨大挑戰。 VYVGART在新適應症或其他經批准的候選產品中的商業化,或者我們的任何產品或候選產品進入新市場將要求我們 進一步擴大我們的銷售和營銷組織,與第三方達成合作安排,將某些功能外包給第三方,或者使用 各自的某種組合。我們已經在VYVGART 批准的某些國家和地區建立並繼續擴大我們的銷售隊伍,並計劃進一步發展我們的銷售和營銷能力,以推廣我們的產品和產品候選,包括新的適應症,如果 在其他相關司法管轄區獲得營銷批准的話。 即使我們成功地擴大了我們的銷售和營銷能力,無論是我們自己還是通過與第三方合作,我們可能無法有效地推出或營銷我們的產品。 招聘和培訓專業銷售人員成本高昂,而且擴大 獨立銷售的成本,營銷和/或促銷組織可能比我們 預期的更大。由於監管措施、停工、停工或罷工、審批延誤、撤回、召回、 處罰、我們所依賴的設施或第三方設施的供應中斷、短缺或缺貨、聲譽損害、流行病或自然災害或 人為災難對我們設施的影響,包括氣候變化、產品責任和/或 意外成本,我們在銷售或營銷方面可能會遇到進一步的困難。此外,招聘和培訓銷售人員非常耗時,而且 可能會推遲任何產品的發佈。如果任何此類發佈因任何原因而延遲或沒有發生 ,我們將過早或不必要地產生這些商業化費用,如果我們無法保留或重新定位我們的銷售和營銷人員,我們的投資將會損失。 我們已與美迪生、再鼎醫藥、Genpharm和韓鐸 簽訂分銷協議,分別在以色列、中東歐、中國大陸中國、GCC和韓國提供銷售和營銷服務。根據這些協議,我們的產品收入或這些產品收入的盈利能力可能低於我們營銷和銷售我們自己開發的產品的情況。此類分銷協議可能會使我們的產品的商業化 超出我們的控制範圍,包括我們的分銷合作伙伴向我們的產品投入資源的數量或時間。此外,我們的 總代理商遵守和完成我們 安排下的義務的意願或能力可能會受到業務合併或 總代理商業務戰略重大變化的不利影響。此外,我們可能無法成功地與第三方達成銷售和營銷我們產品的 安排,或者可能無法按照對我們有利的條款銷售和營銷我們的產品。 我們的產品和候選產品的商業成功,包括新的適應症或給藥方法,將取決於市場接受程度。 我們的產品和候選產品,包括新的適應症或給藥方法,如果獲得批准並在市場上上市,可能永遠不會達到醫生、患者、醫學界和或醫療保健 2.3Argenx資本 共享財務 審核報表 財務非財務 信息 ar Gr g oup enx Factors Risk Go Corporate vernance 2023年年度報告商業化|102 |
| payors for us to be profitable. This will depend on a number of factors, many of which are beyond our control, including, but not limited to: · the efficacy and safety as demonstrated by clinical trials and subsequent prevalence and severity of any side effects; · approval may be for indications, dosage and methods of administration or patient populations that are not as broad as intended or desired; · changes in the standard of care for the targeted indications for any product and product candidate; · availability of alternative approved therapies; · sales, marketing and distribution support; · labeling may require significant use or distribution restrictions or safety warnings; · potential product liability claims; · acceptance by physicians, patients and healthcare payors of each product as safe, effective and cost-effective, and any subsequent changes thereof; · relative convenience, ease of use, including administration, perceived dosing complexity and other perceived advantages over alternative and/or new products; · patient continued commitment required to receive periodic in-center infusions; · prevalence and severity of adverse events discovered before or after marketing approval has been received; · consumer perceptions or publicity regarding our business or the safety and quality of our product and product candidates, clinical trials for new indications, or any similar products distributed by other companies; · limitations, precautions or warnings listed in the summary of product characteristics, patient information leaflet, wording of package labeling or instructions for use, and any subsequent changes thereof; · the cost of treatment with our products in relation to alternative and/or new treatments; · the extent to which products are approved for inclusion and reimbursed on formularies of hospitals and managed care organizations, and any subsequent changes thereof; and · whether our products are designated in the label, under physician treatment guidelines or under reimbursement guidelines as a first-line, second-line, third-line or last-line therapy, and any subsequent changes thereof. In addition, because we are developing our products and product candidates for the treatment of different indications, negative results in a clinical trial evaluating the efficacy and safety of a product or product candidate for one indication could negatively impact the perception of the efficacy and safety of such product or product candidate in a different indication, which could have an adverse effect on our reputation, commercialization efforts and financial condition. Moreover, efforts to educate the medical community and third-party payors on the benefits of our products and product candidates may require significant resources and may never be successful. If our product candidates or methods of use of existing products or new indications fail to gain market acceptance, this will have a material adverse impact on our ability to generate revenues. Even if some products achieve market acceptance, they may not be able to retain market acceptance and/or the market may prove not to be large enough to allow us to generate significant revenues. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 103 |
| We face significant competition for our drug discovery and development efforts. The market for pharmaceutical products is highly competitive and characterized by rapidly growing understanding of disease biology, quickly changing technologies, strong intellectual property barriers to entry, and a multitude of companies involved in the creation, development, and commercialization of novel therapeutics. Many of these companies are highly sophisticated and often strategically collaborate with each other. Competition in the autoimmune field is intense and involves multiple mAbs, other biologics and small molecules either already marketed or in development by many different companies including large pharmaceutical companies such as AbbVie (Humira/rheumatoid arthritis), Amgen, Inc. (Amgen) (Enbrel/rheumatoid arthritis), Biogen Inc, (Tysabr/multiple sclerosis), GlaxoSmithKline plc (GSK) (Benlysta/lupus), F. Hoffman-La Roche AG (Roche) (Rituxan/often used off label) and Janssen Pharmaceuticals, Inc. (Janssen) (Remicade/rheumatoid arthritis and Stelara/psoriasis). In addition, these and other pharmaceutical companies have mAbs or other biologics in clinical development for the treatment of autoimmune diseases. Currently, our commercial revenue is generated by VYVGART, VYVGART HYTRULO and VYVGART SC in gMG. We face and expect to continue to face intense competition from other biopharmaceutical companies, who have launched or are developing products for the treatment of gMG and other autoimmune diseases, including products that are in the same class as VYVGART, as well as products that are similar to some of our product candidates. Competition for other (potential) future indications is also fierce, with significant development in almost all of the indications we are currently developing or planning to develop for our product or product candidates. For example, we are aware of several FcRn inhibitors that are in clinical development and one FcRn inhibitor, Rystiggo (rozanolixizumab-noli), which was approved in June 2023. We are also aware that AstraZeneca PLC is selling Soliris and Ultomiris for the treatment of adult patients with gMG who are AchR-AB+ and that UCB is selling Rystiggo for the treatment of adult patients with gMG who are AchR-AB+ or MuSK-AB+ and Zilbrysq for the treatment of adult patients with gMG who are AchR-AB+. Roche, Novartis AG, CSL Behring, Grifols, S.A., Curavac, Inc., Takeda Pharmaceutical Co Ltd, RemeGen Co, Immunovant, Inc., Cartesian Therapeutics, Inc., Horizon Therapeutics PLC, Regeneron Pharmaceuticals, Inc./Alnylam Pharmaceuticals, Inc. and Johnson & Johnson Innovation, Inc. (Johnson & Johnson) among others, are developing drugs that may have utility for the treatment of MG. Competitive product launches may erode future sales of our products, including our existing products and those currently under development, or result in unanticipated product obsolescence. Such launches continue to occur, and potentially competitive products are in various stages of development. We could also face competition for use of limited international infusion sites, particularly in new markets as competitors launch new products. We cannot predict with accuracy the timing or impact of the introduction of competitive products that treat diseases and conditions like those treated by our products or product candidates. In addition, our competitors and potential competitors compete with us in recruiting and retaining qualified scientific, clinical research and development and management personnel, establishing clinical trial sites, registering patients for clinical trials, as well as in acquiring technologies complementary to, or necessary for, the development of our products. There can be no assurance that our competitors are not currently developing, or will not in the future develop, technologies and products that are equally or more effective, are more economically attractive, and can be administered more easily than any of our current or future technologies or products. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 104 |
| Competing products or technology platforms may gain faster or greater market acceptance than our products or technology platforms and medical advances or rapid technological development by competitors may result in our products and product candidates or technology platforms becoming non-competitive or obsolete before we are able to recover our research and development and commercialization expenses. If we, our products and product candidates or our technology platforms do not compete effectively, it is likely to have a material adverse effect on our business, financial condition and results of operation. Our products and product candidates for which we have obtained or intend to seek approval as biological products, including for new indications, may face biosimilar competition. In the U.S., the BPCIA created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-licensed reference biological product. Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing the sponsor’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. We believe that any of our product candidates approved as a biological product under a BLA should qualify for the 12-year period of exclusivity, as was the case with VYVGART and VYVGART HYTRULO. The regulatory exclusivity periods for VVVGART and VYVGART HYTRULO is expected to extend until December 2033 in the United States. Regulatory protection in the EU (both orphan and data/marketing exclusivity) is expected to expire in August 2032 in the EEA and March 2033 in the UK. Following those periods of regulatory exclusivity, we must enforce our patent rights against biosimilar products that infringe the patent claims of these products. However, there is a risk that this exclusivity could be shortened due to congressional action or otherwise, or that the FDA will not consider our product candidates to be reference products for competing products, potentially creating the opportunity for competition by biosimilar products sooner than anticipated. Moreover, an interchangeable biosimilar product, once approved, may be substituted under existing state law for any one of our reference products in a way that is similar to traditional generic substitution for non-biological products. Any non-interchangeable biosimilar products may also be substituted by a healthcare provider but, under existing law, will not be automatically substituted at the pharmacy. The extent of the impact of such substitution will depend on a number of marketplace and regulatory factors that are still developing. In the EU, biosimilars are evaluated for marketing authorization pursuant to a set of general and product class-specific guidelines. In April 2023, the European Commission adopted a proposal to revise the EU’s pharmaceutical legislation. If adopted in the form proposed, a number of changes to the regulatory framework governing medicinal products in the EEA would occur, including a shortened period of data and market exclusivity. In addition, in an effort to spur biosimilar utilization and/or increase potential healthcare savings, some EU Member States have adopted, or are considering the adoption of, biosimilar uptake measures such as physician prescribing quotas or automatic pharmacy substitution of biosimilars for the corresponding reference products. Some EU Member States impose automatic price reductions upon market entry of one or more biosimilar competitors. In September 2022, the EMA and the EU Heads of Medicines’ Agencies issued a joint statement providing that biosimilar medicines approved in the EU are “interchangeable” with their reference products and other biosimilars of the same reference product. This statement could further contribute to the prescribing of biosimilars and to greater competition in Europe. While the degree of ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 105 |
| competitive effects of biosimilar competition differs among EU Member States and among products, the overall use of biosimilars and the rate at which product sales of innovative products are being affected by biosimilar competition is increasing. Enacted and future legislation could impact demand for our products which could impact our business and future results of operations. In the U.S., the UK, the EU and other jurisdictions, there have been a number of legislative and regulatory changes to the healthcare systems that could affect our future results of operations. Governmental regulations that mandate price controls or limitations on patient access to our products or establish prices paid by government entities or programs for our products could impact our business, and our future results of operations could be adversely affected by changes in such regulations or policies. For example, if the European Commission’s recent proposal to revise the EU’s pharmaceutical legislation is adopted in the form proposed, we may be affected by a decrease in data and market exclusivity for our products and product candidates in the EEA. In particular, there have been and continue to be a number of initiatives at the U.S. federal and state levels that seek to reduce healthcare costs in general and the cost of pharmaceuticals in particular. Healthcare reform initiatives in the U.S. culminated in the enactment of the IRA in August 2022, which allows, among other things, HHS to directly negotiate the selling price of a statutorily specified number of drugs and biologics each year that CMS reimburses under Medicare Part B and Part D. Only high-expenditure single-source biologics that have been approved for at least 11 years (7 years for single-source drugs) can qualify for negotiation, with the negotiated price taking effect two years after the selection year. In August 2023, the U.S. government announced the first 10 drugs to be subject to negotiation, although the Medicare drug price negotiation program is currently subject to legal challenges. Negotiations for Medicare Part D products begin in 2024 with the negotiated price taking effect in 2026, and negotiations for Medicare Part B products begin in 2024 with the negotiated price taking effect in 2028. HHS will announce the negotiated maximum fair price by September 1, 2024, and this price cap, which cannot exceed a statutory ceiling price, will come into effect on January 1, 2026. The IRA also penalizes drug manufacturers that increase prices of Medicare Part D and Part B drugs at a rate greater than the rate of inflation. The IRA will also cap out-of-pocket spending for Medicare Part D enrollees and make other Part D benefit design changes beginning in 2024. Beginning in 2025, the IRA eliminates the coverage gap under Medicare Part D by significantly lowering the enrollee maximum out-of-pocket cost to $2,000 and by requiring manufacturers to subsidize, through a newly established manufacturer discount program, 10% of Part D enrollees’ prescription costs for brand drugs below the out-of-pocket maximum, and 20% once the out-of-pocket maximum has been reached (plans will also be required to cover 20% in this case). Although these discounts represent a lower percentage of enrollees’ costs than the current discounts required below the out-of-pocket maximum (that is, in the coverage gap phase of Part D coverage), the new manufacturer contribution required above the out-of-pocket maximum could be considerable for very high-cost patients and the total contributions by manufacturers to a Part D enrollee’s drug expenses may exceed those currently provided. These Part D design changes may also incentivize Part D plans to exclude certain drugs from their formularies, which could affect the supply, demand, and pricing of our product and product candidates. The IRA permits the Secretary of HHS to implement many of these provisions through guidance, as opposed to regulation, for the initial years. HHS has and will continue to issue and update guidance as these programs are implemented. Manufacturers that fail to comply with the IRA may be subject to various penalties, some significant, including civil monetary penalties. The IRA also extends enhanced subsidies for individuals purchasing health ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 106 |
| 到2025年計劃年ACA市場的保險覆蓋範圍。這些條款從2023年開始逐步生效,儘管它們可能會受到法律挑戰。 例如,與高支出 單一來源藥物和生物製品的銷售價格談判有關的條款已在多起訴訟中受到挑戰,因此,儘管目前尚不清楚愛爾蘭共和軍的全部經濟影響,但該法律的通過可能會影響我們產品和候選產品的定價。此外,作為對拜登政府2022年10月行政命令的迴應,衞生與公眾服務部於2023年2月14日發佈了一份報告, 概述了醫療保險和醫療補助創新中心將測試的三種新模型, 將評估它們降低藥品成本、促進可獲得性和 提高醫療質量的能力。目前還不清楚這些模型是否會在未來的任何醫療改革措施中使用。新司法管轄區採用限制性價格管制、現有司法管轄區採用更嚴格的管制、商業付款人採用這些較低的價格,或未能獲得或維持及時或足夠的定價,也可能對收入造成不利的 影響。我們預計價格壓力將在全球範圍內持續。 此外,在美國州一級,立法機構正在越來越多地制定法律和實施旨在控制藥品和生物製品定價的 法規,包括價格 或報銷限制、折扣要求、營銷成本披露和價格 透明度報告,以及旨在鼓勵從其他國家/地區進口和批量採購的計劃。各州也在以聯邦政策為藍本制定法律,如愛爾蘭共和軍和340B藥品折扣計劃。我們預計未來將採取額外的州和聯邦醫療改革措施,其中任何一項都可能限制聯邦和州政府為醫療產品和服務(包括藥品)支付的 金額,這可能會導致對我們的產品和候選產品的需求減少或額外的定價壓力。 另一方面,歐盟重新開放了 醫療產品的整個立法框架。2023年4月26日,歐盟委員會公佈了其關於制定新的歐盟製藥立法的提案。該提案目前正在歐洲議會和歐盟理事會進行正常的立法程序,因此仍有可能發生變化。如果真的要實施,歐盟製藥立法預計最早將在未來幾年內實施。預防和緩解藥品短缺 ,簡化仿製藥和生物仿製藥的市場準入,減輕監管負擔(例如,通過增加數字化),以及實施針對數據和/或市場獨佔性的新制度 (例如,縮短最短期限,同時引入 因素,如果滿足這些因素,將延長對上市授權持有人的保護),這些都是歐盟委員會追求的 主要目標。在 立法程序結果出來之前,對某些監管 程序的影響可能是積極的。但是,如果不修改 提案,也可能會對Argenx等創新制藥和生物技術公司產生負面影響,因為較短的基線監管和孤立的排他性。 我們受政府定價法律、法規和執法的約束。 這些法律影響我們可能向政府收取的產品價格,以及我們的客户可能從 政府獲得的補償。我們不遵守這些法律可能會損害我們的 結果、運營和/或財務狀況。 在美國,我們必須參加各種政府計劃,才能 獲得聯邦政府的報銷或購買。我們參與了 醫療補助藥品返點計劃、340B藥品折扣計劃、聯邦醫療保險B部分、醫療保險D部分和美國退伍軍人事務部聯邦供應時間表定價計劃 等計劃。要求因計劃而異,但在我們參與的這些計劃和任何其他計劃中,除其他事項外,我們需要與某些政府機構簽訂協議,並計算價格和向某些政府機構報告價格和其他信息,向no ar Gr g oup enx Factors Risk Go Corporate vernance資本收取費用 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告商業化|107 |
| more than statutorily mandated ceiling prices and calculate and pay rebates and refunds for certain products. The calculations are complex and are often subject to interpretation by us, governmental agencies and the courts. If we determine that the prices we reported were in error, we may be required to restate those prices and pay additional rebates or refunds to the extent we understated the rebate or overcharged the government due to the error. Additionally, there are penalties associated with submission of incorrect pricing or other data. We may incur significant civil monetary penalties if we are found to have knowingly submitted false prices or other information to the government, or to have charged 340B covered entities more than the statutorily mandated ceiling price. Certain failures to timely submit required data also could result in a civil monetary penalty for each day the information is late. We could also become subject to allegations under the False Claims Act and other laws and regulations. In addition, misreporting and failure to timely report data to CMS also can be grounds for CMS to terminate our Medicaid rebate agreement, pursuant to which we participate in the Medicaid Drug Rebate Program. In the event that CMS terminates our rebate agreement, no federal payments would be available under Medicaid or Medicare Part B for our covered outpatient drugs. Recently enacted legislation in the U.S. has imposed additional rebates under government programs. For example, effective January 1, 2024, under the American Rescue Plan of 2021, rebates that manufacturers pay to state Medicaid programs was eliminated. Elimination of this cap may require pharmaceutical manufacturers to pay more in Medicaid rebates than they receive on the sale of products for products that have undergone substantial price increases. In addition, the Infrastructure Investment and Jobs Act, effective January 1, 2023, added a requirement for manufacturers of certain single-source drugs (including biologics and biosimilars) separately paid for under Medicare Part B for at least 18 months and marketed in single-dose containers or packages (known as refundable single-dose containers or single-use package drugs) to provide annual refunds for any portions of the dispensed drug that are unused and discarded if those unused or discarded portions exceed an applicable percentage defined by statute or regulation. Manufacturers are subject to periodic audits and those that fail to pay refunds for their refundable single-dose containers or single-use package drugs shall be subject to civil monetary penalties. This requirement applies to VYVGART, and potentially other of our products in the future. As a result, we owe refunds to CMS starting this year. Although we will evaluate options to reduce the amount of refunds owed, pursuing any such actions will be time-consuming and costly. Even if we invest resources to reduce the amount of refunds owed to CMS, it is possible that we will be delayed or unsuccessful in achieving a reduction worthy of our investment. Maintaining compliance with these government price reporting and discounting obligations is time-consuming and costly, and a failure to comply can result in substantial fines, penalties, all of which could adversely impact our financial results. We may not obtain or maintain adequate coverage or reimbursement status for our products and product candidates. Sales of VYVGART for gMG, VYVGART HYTRULO and our product candidates, if approved, will depend, in part, on the extent to which third-party payors, including government health programs in the U.S. (such as Medicare Parts B and D and Medicaid) and other countries, commercial health insurers, and managed care organizations, provide coverage and establish adequate reimbursement levels for such products and product candidates. In the U.S., no uniform policy of coverage and reimbursement for products exists among commercial third-party payors. Commercial third-party payors decide which products they will pay for and establish reimbursement levels. Commercial payors often rely upon Medicare coverage policy and payment limitations in setting their own coverage and reimbursement policies. However, ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 108 |
| decisions regarding the extent of coverage and amount of reimbursement to be provided for any product candidate that we develop through approval will be made on a plan-by-plan basis. One commercial payor’s determination to provide coverage for a product does not assure that other commercial payors will also provide coverage and adequate reimbursement for the product. Additionally, a commercial third-party payor’s decision to provide coverage for a drug does not imply that an adequate reimbursement rate will be approved. Each plan determines whether or not it will provide coverage for a product, what amount it will pay the manufacturer for the product, on what tier of its formulary the product will be placed and whether to require step therapy. The position of a product on a formulary generally determines the co-payment that a patient will need to make to obtain the product and can strongly influence the adoption of a product by patients and physicians. Even under U.S. government healthcare programs such as Medicare and Medicaid, coverage and reimbursement policies can vary significantly. Medicare Part D is administered by commercial insurance companies under contract with the CMS. The many Part D plans operated by these companies vary considerably in their coverage and reimbursement policies, much like the commercial plans that these same companies offer, as described above. Medicare Part B and Medicaid coverage and reimbursement rates are more uniform, but even Medicaid programs vary from state to state in their coverage policies and reimbursement rates. Patients who are prescribed treatments for their conditions and providers prescribing such services generally rely on third-party payors to reimburse all or part of the associated healthcare costs. Patients are unlikely to use our products unless coverage is provided and reimbursement is adequate to cover a significant portion of the cost of our products. Further, from time to time, typically on an annual basis, payment rates are updated and revised by third-party payors. Such updates could impact the demand for our products, to the extent that patients who are prescribed our products, if approved, are not separately reimbursed for the cost of the product. The process for determining whether a third-party payor will provide coverage for a product may be separate from the process for setting the price of a product or for establishing the reimbursement rate that such a payor will pay for the product. Even if we do obtain adequate levels of reimbursement, third-party payors, such as government or private healthcare insurers, carefully review and increasingly question the coverage of, and challenge the prices charged for, products. Increasingly, third-party payors are requiring that biopharmaceutical companies provide them with predetermined discounts from list prices and are challenging the prices charged for products. We may also be required to conduct expensive pharmacoeconomic studies to justify the coverage and the amount of reimbursement for particular medications. We cannot be sure that coverage and reimbursement will be available for any product that we commercialize and, if reimbursement is available, what the level of reimbursement will be. Moreover, coverage policies and third-party payor reimbursement rates may change at any time. Therefore, even if favorable coverage and reimbursement status is attained for one or more products for which we receive marketing approval in one or more indications, less favorable coverage policies and reimbursement rates may be implemented in the future. For instance, even though favorable coverage and reimbursement status has been attained for VYVGART for the treatment of gMG in the U.S., access to VYVGART or for any other indication may be reduced or restricted by limited payer coverage due to treatment criteria, which may prevent us from realizing its full commercial potential. In addition, the coverage and reimbursement levels for our products for the treatment in one indication may have an adverse impact on the coverage and reimbursement levels of such products or product candidates in other indications for which marketing approval has previously been or may subsequently be obtained. Inadequate coverage or reimbursement may diminish or prevent ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Commercialization | 109 |
| 總之,對我們產品的任何重大需求和/或可能會阻止我們完全進入某些市場或適應症,這將阻止我們產生顯著的 收入或實現盈利,這將對我們的業務、財務和 經營業績產生不利影響。 在許多外國國家/地區,處方藥的定價、覆蓋範圍和報銷水平 受政府控制,我們和我們的合作者可能無法獲得 覆蓋範圍、定價、和/或按對我們有利或對我們或我們的合作者有利或必要的條款進行報銷,以使我們或我們的合作者在這些國家/地區成功地銷售我們的市場產品。 在某些外國國家/地區,藥品的建議定價必須獲得批准,然後才能合法銷售。管理藥品定價和報銷的要求因國家/地區而異 ,可能會考慮現有、新藥和新興藥物和治療的臨牀療效、成本和服務 影響。例如,歐盟為其成員國提供了 選項,以限制其國家醫療保險制度為其提供報銷的醫療產品的範圍,並控制 人用醫療產品的價格。成員國可以批准醫藥產品的具體價格,也可以對將醫藥產品投放市場的公司的盈利能力採取直接或間接控制制度。如果我們或我們的合作者無法在國外 銷售我們的產品,或者如果我們在國外銷售的產品的承保範圍和報銷範圍受到限制或延遲,我們的運營結果可能會受到影響。 如果我們未能獲得孤兒藥物指定,或者我們沒有涵蓋我們的產品和候選產品的有效且可執行的專利,並且 無法獲得和/或維持我們產品或候選產品的孤兒藥物排他性,我們的競爭對手可能能夠將產品銷售給 以同樣的條件處理,我們的收入可能會減少。 根據《孤兒藥品法》,在EMA的孤兒藥物產品委員會(COMP)提出建議後,如果一種產品 旨在治療一種罕見的疾病或狀況,在美國的定義是患者人數少於200,000人,或者在美國的患者人數超過200,000人,並且 沒有合理預期開發藥物的成本將從美國在歐盟的銷售中收回,則FDA可將該產品指定為孤兒藥物。歐盟委員會批准孤兒藥物指定,以促進 用於診斷、預防或治療危及生命或慢性衰弱的疾病的產品的開發,這些疾病影響到歐盟每10,000人中不超過5人,或者在沒有激勵措施的情況下,該藥物在歐盟的銷售不太可能足以證明在開發藥物或生物產品方面的必要投資是合理的。在每種情況下,都必須沒有令人滿意的診斷、預防或治療這種疾病的方法,或者,如果存在這種方法,藥物必須對受這種疾病影響的人有顯著的 好處。 在美國,指定孤兒藥物使一方有權獲得經濟激勵,如税收 優惠和用户費用減免。此外,如果一種產品獲得了FDA對其具有孤兒指定的適應症的第一次批准,該產品有權獲得孤兒藥物 獨家經營權,這意味着FDA可能不會批准 另一申請人提交的以相同適應症銷售相同或類似生物製品的任何其他申請,期限為 七年,但在有限情況下除外。一種生物產品是否與另一種產品相同,取決於這兩種產品是否具有相同的主要 分子結構特徵。然而,孤兒指定並不縮短監管審查和審批流程的持續時間。 在歐盟,孤兒藥物指定使一方有權獲得經濟激勵,如降低費用或免除費用,以及在藥品或生物製品獲得批准後獲得10年的市場排他性 。如果孤兒藥物指定標準are ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 Argenx年度報告2023年商業化|110,則這一期限可能縮短至6年 |
| 不再滿足,包括產品被證明足夠有利可圖而不能 證明維持市場排他性是合理的。如果我們無法獲得或失去我們的一個或多個產品和候選產品的孤立藥物狀態 ,我們可能無法或不再獲得上述激勵措施和市場獨佔權,這可能會增加 總體開發成本,並降低此類產品和候選產品(包括生物仿製藥)的競爭地位。類似的考慮也適用於英國。 我們可能會不時地在美國或歐盟為我們的產品和候選產品解決的某些 適應症尋求孤兒藥物稱號。例如,2017年9月,FDA批准將efgartigimod用於GMG的孤兒藥物指定,在批准VYVGART後,FDA授予VYVGART 治療AChR-AB+成年患者GMG的七年孤兒藥物獨家經營權。2022年7月,FDA批准了VYVGART HYTRULO的孤兒藥物名稱,並在VYVGART HYTRULO獲得批准後, FDA授予該產品七年的孤兒藥物獨家經營權,用於治療AChR-AB+成年患者的GMG。2019年1月,FDA批准孤兒藥物 使用efgartigimod治療ITP和使用cuatuzumab治療AML,並於2021年8月批准孤兒藥物 使用與rHuPH20共同配製的efgartigimod治療CIDP。2020年6月,衞生部批准將Efgartigimod用於治療GMG的孤兒藥物指定,並於2022年1月批准VYVGART用於治療GMG。此外,2022年12月,衞生部批准孤兒藥物 使用VYVGART治療ITP。VYVGART治療ITP的批准申請是第一次提交,在全球範圍內是先驅,但這種批准預計將在2024年第一季度獲得。對於我們可能獲得的這些指定或未來的 指定,由於與開發治療性 產品相關的不確定性,我們可能不是第一個獲得此類適應症的 藥物的上市批准,並且我們可能不會在批准後獲得孤立指定。此外,如果我們尋求批准的適應症範圍超過孤兒指定的適應症,則獨家在美國的營銷權可能受到限制,或者如果FDA後來確定指定的請求存在重大缺陷,或者如果我們無法保證 產品的數量足以滿足患有罕見疾病或疾病的患者的需求,則可能會失去獨家營銷權。此外,即使我們獲得了產品的孤立藥物排他性,這種排他性也可能無法有效地保護 產品免受競爭,因為具有不同活性部分或不同主要分子結構特徵的不同藥物可以被批准用於相同的條件。即使在一種孤兒藥物獲得批准後,FDA、EMA或其他外國監管機構也可以隨後批准具有相同主要分子結構特徵的相同藥物用於相同疾病,如果監管機構得出結論認為後者更安全、更有效或對患者護理做出了重大貢獻。 與其他 政府法規相關的風險因素 我們受醫療法律、法規和執法的約束。不遵守這些法律可能會損害我們的業績、運營和/或財務狀況。 我們目前和未來的業務可能是或可能直接或間接通過我們的客户和第三方付款人,受美國聯邦和州、歐盟、日本、中國、英國、加拿大、以色列和其他司法管轄區的醫療保健法律的約束,包括反回扣法規、反賄賂、反腐敗條款、反欺詐法規、虛假索賠行為、 包括AKS、食品、藥品和化粧品法案在內的 。《虛假申報法》等。醫療保健 提供者、醫生和其他人在推薦和處方 我們獲得市場批准的任何產品時扮演主要角色。這些法律可能會影響其他 2.4Argenx Capital 股票財務 審查報表 財務非財務 信息 ar Gr g oup enx Factors Risk Go Corporate vernance年度報告2023年其他政府法規|111 |
| things, our proposed sales, marketing and education programs and constrain our business and financial arrangements with third-party payors, healthcare professionals who participate in our clinical research programs, healthcare professionals and others who recommend, purchase, or provide our approved products, and other parties through which we market, sell and distribute our products for which we obtain marketing approval. In addition, our current and future operations are subject to other healthcare-related statutory and regulatory requirements and enforcement by regulatory authorities in jurisdictions in which we conduct our business. For example, the provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order or use of medical products is generally not permitted in countries that form part of the EU, or the UK. Some EU Member States have enacted laws explicitly prohibiting the provision of these types of benefits and advantages to induce or reward improper performance generally, and the UK has enacted similar restrictions through the Bribery Act 2010. Infringements of these laws can result in substantial fines and imprisonment, as well as associated reputational harm. We are also subject to EU Directive 2001/83/EC and the Human Medicines Regulations 2012. Any action against us for violation of these laws, even if we successfully defend against it, could cause us to incur significant legal expenses and divert our management’s attention from the operation of our business. The shifting compliance environment and the need to maintain robust and expandable systems to comply with multiple jurisdictions with different compliance or reporting requirements increases the possibility that we or our collaborative partners may run afoul of one or more of the requirements. We continue to expand, enhance and refine our internal ethics and compliance function and program to ensure compliance with the different healthcare laws and regulations. The expansion and maintenance of an internal compliance program involves substantial costs and, notwithstanding our investment, mechanisms put in place to ensure compliance with applicable laws and regulations and our best efforts, the program may not be fully successful as there can be no assurance that our policies and procedures will be followed at all times or will effectively detect and/or prevent all compliance violations by our employees, consultants, subcontractors, agents and partners. It is possible that governmental authorities will conclude that our business practices do not comply with current or future statutes, regulations or case law involving applicable fraud and abuse or other healthcare laws and regulations. If our operations are found to be in violation of any of these laws or any other governmental regulations that may apply to us, we may be subject to significant civil, criminal and administrative investigations, penalties, damages, fines, disgorgement, imprisonment, exclusion of drugs from government funded healthcare programs, such as Medicare and Medicaid in the U.S., additional reporting requirements and oversight if we become subject to a corporate integrity agreement or similar agreement to resolve allegations of non-compliance with these laws, reputational harm and the curtailment or restructuring of our operations. Managing such investigations and defending against or appealing any such actions or penalties can be costly and time-consuming and may require significant financial and personnel resources. Therefore, even if we are successful in managing any such governmental investigations and/or defending against or appealing any such actions or penalties that may be brought against or imposed upon us, our business may be impaired. Further, if any of the physicians or other healthcare providers or entities with whom we expect to do business is found to be not in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including exclusions from government funded healthcare programs. Efforts to ensure that our business arrangements with third parties comply with applicable healthcare laws and regulations also involves substantial costs. The scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform. Federal and state enforcement bodies have ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Government Regulations | 112 |
| recently increased their scrutiny of interactions between healthcare companies and healthcare providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry. Ensuring business arrangements comply with applicable healthcare laws, as well as responding to possible investigations by government authorities, can be time and resource consuming and can divert a company’s attention from the business. All aspects of our business ranging from preclinical, clinical trials, marketing and commercialization are highly regulated and any delay by relevant regulatory authorities could jeopardize our development and approval process or result in other suspensions, refusals or withdrawal of approvals. Before we can commence clinical trials for a product candidate, we must complete extensive preclinical testing and clinical trials that support our IND or planned IND applications in the U.S. or Japan, or our CTAs in the UK or in the EU, or a comparable application in other jurisdictions. We cannot be sure that we will be able to submit INDs or CTAs or comparable applications for our preclinical programs on the timelines we expect, if at all. We also cannot guarantee that submission of INDs or CTAs or comparable applications will result in the MHRA, EMA, FDA, MHLW (collectively, the Relevant Regulatory Authorities) or other regulatory authorities allowing clinical trials to even begin. Clinical trials must be conducted in accordance with Relevant Regulatory Authorities and other applicable regulatory authorities’ legal requirements and regulations and are subject to oversight by these governmental agencies and IRBs and ethics committees at the medical institutions where the clinical trials are conducted. In addition, clinical trials must be conducted in compliance with GCPs and with supplies of our products and product candidates produced under cGMPs and other regulations. We could encounter delays if a clinical trial is suspended or terminated, by us, by the IRBs or ethics committees of the institutions in which such clinical trials are being conducted, by the data review committee or data safety monitoring board for such clinical trial by the Relevant Regulatory Authorities or other comparable regulatory authorities. Such authorities may impose a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or clinical trial site by the Relevant Regulatory Authorities or other applicable authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, including those relating to the class to which our products and product candidates belong, failure to demonstrate a benefit from using the product or product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If we experience delays in the completion of, or termination of, any clinical trial of our products or product candidates, the costs of our clinical trials may increase, the commercial prospects of our products and product candidates may be harmed, and our ability to generate product revenues from any of these products and product candidates will be delayed. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our products and product candidates. Moreover, we must obtain separate regulatory approvals in each jurisdiction where we want to market and approval by one regulatory authority does not ensure approval by any other regulatory authority. As approval procedures can vary among countries and may change over time, this can require additional clinical testing and the time required to obtain approval may differ. We can provide no assurances that such approval will be obtained on the timeline that we expect or at all. In addition, we anticipate to submit applications for approval of VYVGART ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Government Regulations | 113 |
| in new indications, but can provide no assurances that such applications will be accepted or that we will receive approval on our anticipated timeline, or at all. If VYVGART or any new formulations of VYVGART are not approved in one or more jurisdictions including beyond the VYVGART Approved Countries, or if such approvals are significantly delayed, it could have a material adverse effect on our business. It is possible that none of our other existing product candidates or any product candidates we may seek to develop in the future will ever obtain regulatory approval in any other jurisdiction or indication. Further, Relevant Regulatory Authorities may impose extensive and ongoing unique regulatory requirements. For example, they: · may withdraw an approval or revoke a license; · may refuse to grant approval, or may require additional data before granting approval, notwithstanding that approval may have been granted by another comparable foreign authority; · may approve a product candidate for fewer or more limited indications or patient sub-segments than requested; or · may grant approval contingent on the performance of costly post-marketing clinical trials, including Phase 4 clinical trials, and surveillance to monitor the safety and efficacy of the product candidate; or · may approve a product candidate with a label that does not include the labeling claims necessary or desirable for the successful commercialization of that product candidate. The costs of compliance with all Relevant Regulatory Authorities and applicable authorities regulations, requirements or guidelines could be substantial, and failure to comply could result in sanctions, including fines, injunctions, civil penalties, denial of applications for marketing authorization of our products, delays, suspension or withdrawal of approvals, license revocation, seizures or recalls of products, operating restrictions and criminal prosecutions, any of which could significantly increase our and/or our collaborative partners’ costs or delay the development and commercialization of our product candidates. At this time, we cannot guarantee or know the exact nature, precise timing and detailed costs of the efforts that will be necessary to complete the remainder of the development of our research programs and product candidates. We are subject to privacy laws, regulation and potential enforcement. Our failure to comply with these laws could harm our results, operations and/or financial conditions. Privacy laws, regulation and potential enforcement are particularly relevant to our business as we collect, store and process patient data, including sensitive health data as well as human biological samples such as blood or tissue, in the context of our clinical development activities, post-marketing approval monitoring obligations, and associated activities. We also collaborate on a regular basis with third parties where we may seek to use data collected by third parties on our or their behalf, or we may seek to share data collected by us with such third parties to further our research or commercial initiatives. The GDPR imposes a broad range of strict requirements on companies, including with respect to cross-border transfers of personal data. The GDPR allows the imposition of substantial penalties in the event of non-compliance, including fines of up to €10 million or up to 2% of total worldwide annual turnover of the preceding fiscal year for certain comparatively minor offenses, or up to €20 million or up to 4% of total worldwide annual turnover if the preceding fiscal year for more serious offenses. We face uncertainty as to the exact interpretation of the ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Government Regulations | 114 |
| 我們可能無法成功實施數據保護當局或法院在解釋GDPR時所要求的所有措施。 此外,歐盟成員國的國家法律可能會部分偏離GDPR,並因國家/地區的不同而施加不同的義務,因此我們不會在歐盟統一的 法律環境中運營。此外,在處理基因數據方面,GDPR明確允許歐盟成員國的法律施加額外和更具體的要求或限制,而歐洲各國法律在這一領域歷來存在很大差異,導致了額外的不確定性。 脱離歐盟後,英國保持了與GDPR(英國GDPR)基本等同的 條款。英國政府最近宣佈對政權進行改革。我們對英國GDPR和其他英國數據保護規則的遵守情況與上述問題類似。 隱私法在歐洲繼續發展和擴展。例如,歐洲議會和歐洲理事會2002年7月12日的第2002/58/EC號指令(修訂後的電子隱私指令)要求歐盟成員國實施法律,以滿足與電子通信、Cookie和在線監控以及其他數字隱私相關的嚴格隱私要求。違反這些要求可能會導致行政措施,包括罰款或刑事處罰。歐盟正在制定新的電子隱私法規以取代電子隱私指令,新的電子隱私法規可能會對我們的業務施加 額外的義務和風險。 在歐盟和英國之外,隱私和數據保護法律和法規繼續在世界各地發展和擴展,包括在我們開展業務的其他司法管轄區,如美國、日本和加拿大。此類法律和法規對個人數據的處理施加了越來越多的限制和義務,包括 基因數據等敏感個人數據。例如,在美國,經經濟和臨牀健康信息技術 和臨牀健康法案修訂的1996年聯邦醫療保險可攜帶性和責任法案對可單獨識別的健康信息的隱私、安全和傳輸提出了具體要求,以及新的州隱私法律,如2018年加州消費者隱私法(CCPA)和2023年華盛頓我的健康我的數據 法案,對承保企業施加了義務,包括但不限於,在隱私通知中提供特定披露,並向居民提供與其 個人數據相關的某些權利。此外,自2023年1月1日起,加州2020年隱私權法案(CPRA)對立法涵蓋的公司施加了額外的義務,並將 大幅修改CCPA,包括擴大消費者對某些敏感個人信息的權利。CPRA還創建了一個新的國家機構,將被授予 實施和執行CCPA和CPRA的權力。此外,在美國,一些州也頒佈了類似的法律,許多其他州也提出了類似的法律。 CCPA和CPRA的影響可能很大,可能需要我們修改我們的數據收集或處理實踐和政策,並在努力遵守和增加我們在監管執法和/或訴訟中的潛在風險方面產生巨大的成本和支出。如果我們受到數據保護機構的調查,我們可能面臨罰款和其他 處罰。此類數據保護機構的任何此類調查或指控都可能對我們現有的業務以及我們吸引和留住新客户或醫藥合作伙伴的能力產生 負面影響。我們還可能遇到客户 或製藥合作伙伴因某些數據保護機構在解釋現行法律時強加給他們的當前(尤其是未來)數據保護義務 可能導致的風險敞口而猶豫、不願或拒絕繼續使用我們的產品和解決方案。此類 客户或醫藥合作伙伴也可能認為任何替代合規方法 成本太高、負擔太重、法律上太不確定或令人反感,因此 決定不與我們做生意。上述任何一項都可能損害我們的業務、 operations. ar Gr g oup enx Factors Risk Go Corporate vernance資本的前景、財務狀況和業績 股票財務 審查報表 財務非財務 信息 Argenx年度報告2023年其他政府法規|115 |
| 如果不遵守反腐敗法律法規、反洗錢法律法規、經濟制裁和/或出口管制法規以及管理我們業務的其他法律,例如與可持續性有關的 ,可能會對我們的業務產生不利影響。 我們正在或可能會受到多個司法管轄區發佈的有關反腐敗、反洗錢、經濟制裁、投資限制、反欺詐和出口管制的各種法律法規的約束。其中包括英國2010年《反賄賂法》和修訂後的美國1977年《反海外腐敗法》,該法禁止以不正當方式影響外國官員的任何行為或決策為目的的付款、要約或承諾。我們的業務性質意味着我們與外國官員進行重大互動。我們還受到美國財政部外國資產控制辦公室、美國政府其他機構、英國財政部、歐盟和聯合國等機構實施的經濟制裁和出口管制規則和法規的約束。進出口法規、經濟制裁法規或相關法規的任何 變化、現有法規執行或範圍的變化,或此類法規針對的國家/地區、政府、個人或技術的變化,都可能降低我們在國際上製造、進口、出口或銷售我們產品的能力。對我們產品製造、進口、出口或銷售能力的任何限制都可能對我們的業務產生不利影響。 我們已建立機制來確保遵守此類規章制度。 但是,不能保證我們的政策和程序在所有 次都會得到遵守,也不能保證有效地檢測和/或防止我們的員工、顧問、分包商、代理商和合作夥伴 違反適用的合規制度。因此,如果發生不遵守規定的情況,我們可能會受到重大的民事或刑事處罰,包括對我們的經濟制裁,對負責任的員工和經理的監禁, 可能失去的進出口特權,聲譽損害,以及由此造成的收入和利潤損失 ,這可能對我們的業務、財務狀況和運營產生實質性的不利影響。 此外,越來越多的投資者、監管機構、自律組織和其他 利益相關者已表示有興趣設置環境,社會和治理 (ESG)目標,並要求提供新的和更有力的披露為實現這些目標而採取的步驟。歐盟的相關立法格局也在相應地發展 。例如,2023年1月5日,關於企業可持續發展報告(CSRD)的歐洲議會和理事會2022年12月14日修訂(EU)第537/2014號條例的(EU)2022/2464號指令、 2004/109/EC號指令、2006/43/EC號指令和2013/34/EU號指令生效。這一新指令加強了有關公司必須報告的社會和環境信息的規則。它擴大了 公司報告ESG信息的範圍,並增加了所需披露的深度。CSRD還引入了“雙重重要性”分析,要求 公司報告可持續性問題可能如何給公司造成財務風險,以及公司自身對人員和環境的影響。CSRD適用於歐盟大型公司、“大型集團”的歐盟母公司和歐盟上市的中小型公司 。它還適用於符合特定標準的非歐盟公司,包括歐盟 營業額門檻和歐盟分公司或子公司。要報告的具體信息 在歐洲可持續發展報告標準(ESRS)中規定。受 CSRD約束的公司將必須根據其類別交錯遵守報告要求。對於符合歐盟大型公司資格且已經 有義務發佈非財務報表的上市公司,將需要在2024年1月1日或之後的財政年度根據ESRS 發佈強制性可持續發展報告。這 意味着在2025年,我們將首次發佈一份符合CSRD要求的可持續發展報告,該報告將整合到我們2024年財政年度報告中。 荷蘭政府正在將CSRD實施到荷蘭legislation. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查聲明 財務非財務 信息 argenx年度報告2023年其他政府法規|116 |
| This means that in 2025, we will have to publish our first CSRD report covering the prescribed ESG information for the financial year 2024. The Dutch government is in the process of implementing the CSRD into Dutch legislation. In July 2023, a Dutch draft bill to implement certain elements of the CSRD (including the requirements for assurance of CSRD reports and applicability to listed companies) was published and submitted for public consultation, which consultation period ended in September 2023. Additionally, in November 2023, a Dutch draft decree implementing certain elements of the CSRD (including the CSRD disclosure obligations for in-scope companies, assurance rules and implementation timelines) was published and submitted for public consultation, which consultation period ended in December 2023. The Dutch government will need to progress the parliamentary debate and adoption of the aforementioned draft bill and decree in the near future given that the ultimate date for implementation of the CSRD within EU member states at a local level is July 6, 2024. Similarly, the SEC adopted on March 6, 2024 final rules aimed at enhancing and standardizing climate-related disclosures relating to climate-related risks, Scope 1 and Scope 2 greenhouse gas emissions and climate-related financial metrics (SEC Climate Rules). As an foreign private issuer and large accelerated filer, we will need to begin complying with the disclosure requirements of the SEC Climate Rules in our Form 20-F for the fiscal year ending December 31, 2025, which will include quantitative and qualitative climate-related disclosures. In response to new ESG initiatives and regulations we may voluntarily elect, or be required, to adopt strategies, policies, or procedures related to ESG matters. Reporting on ESG goals and objectives, including pursuant to the SEC Climate Rules, may cause us to expend significant capital and human resources, and could divert management’s attention from central operational matters. Reports could also lead to the disclosure of information that which may have a negative impact on our operations and reputation which may lead to additional exposure. Failure to accurately comply with any ESG reporting obligations may result in enforcement actions, sanctions, reputational harm or private litigation. We may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities. Our operations, including our research, development, testing and third-party manufacturing activities, are subject to numerous environmental, health and safety laws and regulations and for which we may become liable. If we or one of our contract manufacturing organizations (CMOs) or third-party distributors, manufacturers, licensees or co-marketers fail to comply with such laws and regulations, such failure could result in substantial fines, penalties or other sanctions which could also bring significant reputational loss to our business. We face a risk of environmental liability inherent in our current and historical activities, including liability relating to releases of our exposure to hazardous or biological materials. Furthermore, environmental, health and safety laws and regulations are becoming more stringent. Both us and our CMOs may be required to incur substantial expenses in connection with future environmental compliance or remediation activities, in which case, our production and development efforts may be interrupted or delayed, and our financial condition and results of operations may be materially adversely affected. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Government Regulations | 117 |
| Risk Factors Related to the Development and Clinical Testing of argenx’s Products and Product Candidates Failure to successfully identify, select and develop VYVGART in other indications, or additional products or product candidates could impair our ability to grow. Our long-term growth strategy entails developing and marketing additional products and product candidates, including VYVGART in new indications, which requires substantial resources, whether or not any product candidates or new indications are ultimately identified. The success of this strategy depends partly upon our ability to identify, select, develop, and ultimately, commercialize promising product candidates. We are heavily dependent on precise, accurate and reliable scientific data to identify, select and develop promising product candidates and products. Our business decisions may therefore be adversely influenced by inaccurate, improper or fraudulent scientific data, including data sourced from third parties. Any irregularities in the scientific data used by us to determine our focus in research and development of product and product candidates, could impair our ability to grow. Even with accurate scientific data, our technology platforms may fail to discover and to generate additional products and products candidates, that are suitable for further development. Even if we identify additional product candidates, they may not be suitable for clinical development as a result of harmful side effects, limited efficacy or other characteristics that indicate that it is unlikely to be a product that will receive approval by the Relevant Regulatory Authorities and other comparable regulatory authorities or achieve market acceptance. For example, in November 2023, we announced that our ADVANCE-SC clinical trial evaluating VYVGART HYTRULO in adults with ITP did not meet the primary endpoint of a sustained platelet count response in chronic ITP patients. Secondary endpoints were also not met. In December 2023, we announced that topline data from the Phase 3 ADDRESS clinical trial evaluating SC efgartigimod in adults with PV and PF showed that the proportion of PV patients achieving the primary endpoint of complete remission on CRmin was not significantly different between SC efgartigimod and placebo. We consequently decided not to pursue additional development in pemphigus and plan to prioritize clinical development of efgartigimod in its ongoing severe autoimmune indications. If we do not successfully identify, develop and commercialize product candidates and VYVGART in new indications based upon our technological approach, we may not be able to obtain product or collaboration revenues in future periods. VYVGART has obtained regulatory approval in the VYVGART Approved Countries for the treatment of gMG. Our other products and product candidates – including additional indications or methods of use for efgartigimod, empasiprubart and ARGX-119 – are either in preclinical or clinical development or are pending marketing approval. To obtain the requisite regulatory approvals to market and sell any of our products and product candidates, we or our collaborators for such candidates must successfully demonstrate that our products are safe and effective in humans. Clinical trials are expensive and can take many years to complete, and their outcome is inherently uncertain. Further, success in early clinical trials or in one indication does not guarantee success in later clinical trials or in other indications. 2.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Development and Clinical Testing | 118 |
| The time required to obtain approval by the Relevant Regulatory Authorities is unpredictable but typically takes many years, if obtained at all, following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion or interpretation of the regulatory authorities. This lengthy approval process as well as the unpredictability of future clinical trial results may result in our failing to obtain regulatory approval to market any of our product candidates, including for new indications. We may experience delays in our ongoing or planned clinical trials, for a large variety of reasons outside our control in complying with regulatory approvals which can adversely affect the timing of trials, including as described in the section 2.4.2 “All aspects of our business ranging from preclinical, clinical trials, marketing and commercialization are highly regulated and any delay by relevant regulatory authorities could jeopardize our development and approval process or result in other suspensions, refusals or withdrawal of approvals.” If we are unable to obtain regulatory approval of our products and product candidates on a timely basis or at all, our business may be impacted. Our clinical trials have, and may in the future, fail, and even if they succeed, we may not obtain regulatory approval for our products and product candidates or regulatory approval may be delayed. Even if clinical trials are initiated, our development efforts may not be successful. Many of our clinical trials are blinded, which may cause us to incur significant expenses without any visibility as to the likelihood of successful results. Even if we obtain positive results from preclinical trials or initial clinical trials, we may not achieve the same success in future clinical trials. Regulatory approval of our products or product candidates may be delayed or refused for many reasons, including: · the Relevant Regulatory Authorities may disagree with the design or implementation of our clinical trials; · we may be unable to demonstrate, to the satisfaction of the FDA or comparable foreign regulatory authorities, that our product candidates are safe, pure, potent and effective for any of their proposed indications; · we may be unable to demonstrate our product candidates’ clinical and other benefits outweigh their safety risks; · the FDA may determine that clinical trial results are not generalizable to the U.S. population and/or U.S. medical practice based on the proportion and results of subjects outside of the U.S. where differences in patient management might affect the treatment response. Comparable foreign regulatory authorities may take a similar approach; · the results of clinical trials may not meet the level of statistical significance required by the FDA or comparable foreign regulatory authorities for approval; · the chemistry, manufacturing and controls information submitted in a marketing application is insufficient; and · the facilities of third-party manufacturers with which we contract for the manufacture of our product candidates are not adequate to support approval of our product candidates. Any of these occurrences may harm our business, results of operations and financial condition significantly. We could also experience operational challenges as we undertake an increasing number of clinical trials, including those conducted in countries outside the EU, UK and the U.S. that may subject us to further delays and expenses as a result of increased shipment costs, additional regulatory requirements and the engagement of non-EU, non-UK and non-U.S. contract ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Development and Clinical Testing | 119 |
| research organizations (CROs), as well as expose us to risks associated with clinical investigators who are unknown to the Relevant Regulatory Authorities, and apply different standards of diagnosis, screening and medical care. If we experience delays in the completion of, or termination of, any clinical trial of our products or product candidates, our commercial prospects may be harmed. Any delays in completing our clinical trials may increase our costs, slow down our product candidate development and approval process and jeopardize our ability to commence product sales and generate revenues. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates or result in the development of our product candidates being stopped early. Significant clinical trial delays could also allow our competitors to bring products to market before we do or shorten any periods during which we have the exclusive right to commercialize our products and product candidates. If we decide to pursue accelerated approval for any of our product candidates, it may not lead to a faster development or regulatory review or approval process and does not increase the likelihood that our product candidates will receive marketing approval. If we are unable to obtain approval under an accelerated pathway, we may be required to conduct additional clinical trials beyond those that we contemplate, which could increase the expense of obtaining, reduce the likelihood of obtaining, and/or delay the timing of obtaining, necessary marketing approvals. In the future, we may decide to pursue accelerated approval for one or more of our product candidates. For example, under the FDA’s accelerated approval program, the FDA may approve a drug or biological product for a serious or life-threatening disease or condition that provides a meaningful advantage over available therapies based upon a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit. For products granted accelerated approval, post-marketing confirmatory clinical trials are required to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. These confirmatory clinical trials must be completed with due diligence, and the FDA may require that the clinical trial be designed, initiated, and/or fully enrolled prior to approval. If we were to pursue accelerated approval for a product candidate for a disease or condition, we would do so on the basis that there is no available therapy for that disease or condition. If standard of care were to evolve or if any of our competitors were to receive full approval on the basis of a confirmatory clinical trial for a drug or biological product for a disease or condition for which we are seeking accelerated approval before we receive accelerated approval, the disease or condition may no longer qualify as one for which there is no available therapy, and accelerated approval of our product candidate may not occur. Moreover, the FDA may withdraw approval of any product candidate approved under the accelerated approval pathway if, for example: · the clinical trial or clinical trials required to verify the predicted clinical benefit of our product candidate fail to verify such benefit or do not demonstrate sufficient clinical benefit to justify the risks associated with such product; · other evidence demonstrates that our product candidate is not shown to be safe or effective under the conditions of use; · we fail to conduct any required post-approval trial of our product candidate with due diligence; or ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Development and Clinical Testing | 120 |
| · we disseminate false or misleading promotional materials relating to the relevant product candidate. Recently, the accelerated approval pathway has come under scrutiny within the FDA and by Congress. The FDA has put increased focus on ensuring that confirmatory studies are conducted with diligence and, ultimately, that such studies confirm the benefit. The recently enacted FDORA legislation includes provisions related to the accelerated approval pathway. Pursuant to FDORA, the FDA is authorized to require a post-approval clinical trial to be underway prior to approval or within a specified time period following approval. FDORA also requires the FDA to specify conditions of any required post-approval clinical trial and requires sponsors to submit progress reports for required post-approval studies. In addition, FDORA enables the FDA to initiate enforcement action for the failure to conduct with due diligence a required post-approval clinical trial, including a failure to meet any required conditions specified by the FDA or to submit timely reports. Failure to obtain accelerated approval for our product candidates could result in a longer time period to commercialization of such product candidate, if any, and could increase the cost of development of such product candidate and harm our competitive position in the marketplace. Our products and product candidates may have serious adverse, undesirable or unacceptable side effects or even cause death, and we or others may identify undesirable or unacceptable side effects caused by VYVGART or any of our products or product candidates after they have received marketing approval. Undesirable side effects that may be caused by our product candidates, or by the combination of our product candidates with other medical products, could cause us or regulatory authorities to interrupt, delay or halt clinical trials and could result in more restrictive labeling or the delay or denial of regulatory approval by the Relevant Regulatory Authorities. While our preclinical trials and clinical trials for our product candidates to date show that our product candidates have generally been well tolerated from a risk-benefit perspective, we have observed adverse events and treatment emergent adverse events (TEAEs) in our clinical trials to date, and we may see additional adverse events and TEAEs in our ongoing and future clinical trials. Such side effects may be more serious than those observed to date, and as a result, our ongoing and future clinical trials may be negatively impacted. Moreover, as we seek to develop product candidates, including products in new indications, patients may experience new or more serious effects. Drug-related side effects could affect patient recruitment, the ability of enrolled patients to complete the clinical trial, result in potential product liability claims, damage sales of our existing products, result in significant reputational damage for us and our product development, and other issues including the delay of other programs. Additionally, if we or others identify undesirable or unacceptable side effects caused by VYVGART or any of our other product candidates after they receive marketing approval, a number of potentially significant negative consequences could arise, including: · regulatory authorities may withdraw approvals or revoke licenses of such products and require us to take such products off the market; · regulatory authorities may require the addition of labeling statements, specific warnings, or a contraindication or request the issuance of field alerts to physicians and pharmacies; · regulatory authorities may require a medication guide outlining the risks of such side effects for distribution to patients, or a REMS plan to ensure that the benefits of the product outweigh its risks; · we may be required to change the way the product is administered, conduct additional clinical trials or change the labeling of the product; ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Development and Clinical Testing | 121 |
| ·我們可能會受到如何推廣產品的限制; ·產品的銷量可能會大幅下降; ·我們可能會受到訴訟、產品責任索賠或刑事起訴;以及 ·我們的聲譽可能會受到影響。 任何這些事件都可能對我們、我們的合作者或我們潛在的未來合作伙伴產生負面影響 。此外,相關監管機構可能要求更改標籤甚至吊銷許可證,這可能會損害我們的聲譽,並對我們將VYVGART商業化的能力 產生重大不利影響。 如果我們的目標患者人數少於預期,我們無法 在我們的臨牀試驗中成功招收和留住患者,或者 在這方面遇到重大延誤,我們可能無法充分發揮任何產品或候選產品的商業潛力。 目前,我們主要開發用於治療目標患者人數可能較少的罕見 疾病的產品或候選產品。如果這些疾病的實際患者數量 少於我們的預期,我們可能會在臨牀試驗中招募足夠的患者時遇到困難,從而延遲或阻止我們的產品或候選產品的開發和 批准。醫生是轉介患者進行臨牀試驗的重要來源,他們也可能對這些罕見疾病不太熟悉,因此可能無法識別患者的這些情況,因此可能不會將他們推薦給我們的臨牀試驗。 患者登記是臨牀試驗時間安排的一個重要因素,取決於許多因素, 包括患者羣體的規模和性質、臨牀試驗的資格標準、患者離臨牀地點的距離、從競爭性臨牀試驗中招募患者的競爭、臨牀方案的設計、臨牀試驗的資格標準,臨牀試驗正在調查的適應症的替代批准療法的可用性,以及臨牀醫生和患者對正在研究的藥物相對於其他可用療法的潛在優勢的看法。我們與其他公司競爭招募 目標患者羣體,如風險因素第2.3.3節所述,在我們的藥物發現和開發工作中,我們面臨着激烈的 競爭。即使候選產品 因其批准的適應症而獲得相當大的市場份額,但由於某些潛在目標人羣較少,如果未獲得監管部門對此類候選產品的額外適應症的批准,我們可能永遠無法收回在此類候選產品上的投資。 即使註冊後,我們也可能無法留住足夠數量的患者來完成我們的任何臨牀試驗。此外,我們可能在候選藥物的臨牀試驗中報告的任何負面結果可能會使我們很難或不可能在同一候選藥物的其他 臨牀試驗中招募和留住患者。延遲完成我們的 候選產品的任何臨牀試驗將增加我們的成本,減緩我們的候選產品開發 和審批流程,並延遲或潛在地危及我們開始銷售產品和創造收入的能力。此外,一些導致或導致臨牀試驗延遲開始或完成的因素也可能最終導致我們的候選產品無法獲得監管部門的批准。 此外,我們臨牀試驗中登記的某些患者位於 衝突、敵對行動或戰爭或我們無法控制的其他破壞性事件的地區。見第2.9.2節“全球地緣和社會政治威脅、宏觀經濟不確定性以及其他不可預見的政治危機可能對我們的業務和財務業績產生重大不利影響。”以及“我們面臨着與自然災害和公共衞生相關的風險 這些問題可能會對我們的業務和財務產生負面影響condition”. ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 argenx 2023年年度報告開發和臨牀測試|122 |
| Risk Factors Related to argenx’s Dependence on Third Parties We rely, and expect to continue to rely, on third parties to conduct some of our research activities and clinical trials and for parts of the development and commercialization of our existing and future research programs, products and product candidates. If our relationships with such third parties are not successful, our business may be adversely affected. We have relied upon and plan to continue to rely upon third parties, including independent clinical investigators, CROs, CMOs and other third-party service providers, to assist us in the conduct of certain of our research activities and clinical trials and to monitor and manage data for our ongoing preclinical trials and clinical trials. We also depend on our collaborators and on medical institutions and CROs to conduct our research activities and clinical trials in compliance with regulatory and legal requirements, including GCPs or GMPs, our standard operating procedures and our applicable protocols. Nevertheless, we are responsible for ensuring that each of our preclinical trials and clinical trials is conducted in accordance with the applicable protocol, legal and regulatory requirements and scientific standards, and our reliance on these third parties does not relieve us of our regulatory responsibilities. To the extent our collaborators or the CROs or investigators fail to enroll participants for our clinical trials, fail to conduct the clinical trial to GCP standards or in full compliance with legal and regulatory requirements or are delayed for a significant time in the execution of clinical trials, including achieving full enrollment, we may be affected by increased costs, program delays or both, which may harm our business. In addition, we are, and expect to continue to be, dependent on partnerships with partners and licensees relating to the development and commercialization of our existing and future research programs, products and product candidates. We currently have collaborative research relationships with various pharmaceutical companies such as AbbVie, Zai Lab, Genmab and with various academic and research institutions worldwide for the development of product candidates resulting from such collaborations. We also have distribution agreements with Medison, Genpharm and Handok for the distribution of VYVGART. We had, have and will continue to have discussions on potential partnering opportunities with various pharmaceutical companies. If we fail to enter into or maintain collaborations on reasonable terms or at all, our ability to develop our existing or future research programs and product candidates and to commercialize our existing or future products could be delayed, the commercial potential of our products could change and our costs of development and commercialization could increase. While we have agreements governing our relationships with these third parties, we have limited influence over their actual performance and control only certain aspects of their activities. If independent investigators, third-party service providers or CROs fail to devote sufficient resources to the development of our product candidates, or if their performance is substandard, it may delay or compromise the prospects for approval and commercialization of any product candidates that we develop. In addition, regulatory authorities enforce these GCPs through periodic inspections of trial sponsors, principal investigators and trial sites. If we, our investigators or any of our CROs fail to comply with applicable GCPs, the clinical data generated in our clinical trials may be deemed unreliable and the Relevant Regulatory Authorities or comparable regulatory authorities may require us to perform additional clinical trials before approving our marketing applications. Upon inspection by a given regulatory authority, such regulatory authority may determine that our clinical trials do not fully comply with GCP regulations, which may require us to repeat clinical trials and delay the regulatory 2.6 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Dependence on Third Parties | 123 |
| approval process. Our collaborative partners may not adhere or terminate collaboration agreements with all associated consequences or disagree on the interpretation of contractual terms. We may not be able to control our collaborative partners’ compliance with all applicable requirements for the commercialization of our products, which could adversely affect such commercialization and the profitability of such products. Failures by our collaborative partners to meet their contractual, regulatory, or other obligations to us or any disruption in the relationships between us and our collaborative partners, could have a material adverse effect on our product pipeline and business. We face significant competition in establishing successful relationships with third-party service providers and appropriate collaborative partners. These third-party service providers may have contractual relationships with other entities, some of which may be our competitors, which may draw their time and resources away from our programs. In addition, some of our third-party service providers or CROs have the ability to terminate their respective agreements with us, and if such agreements terminate, we may not be able to enter into arrangements with alternative CROs or investigators or to do so on commercially reasonable terms. In addition, we may not be able to find appropriate collaboration partners. Our ability to reach a definitive agreement for a partnership will depend, among other things, upon an assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed partnership and the proposed collaborator’s evaluation of a number of factors. These factors may include the design or results of clinical trials, the likelihood of regulatory approval, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership regardless of the merits of the challenge and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a partnership could be more attractive than the one with us. Disruptions caused by our reliance on third parties for our manufacturing process may delay or disrupt our business, product development and commercialization efforts. We do not have the ability to internally source the raw materials necessary to produce our products or product candidates, and do not currently have, nor do we plan to acquire, the infrastructure or capability internally to manufacture our products or product candidates and depend on a worldwide supply chain and third parties for both. Disruptions caused by our reliance on such third-party suppliers, service providers and manufacturers may delay or disrupt our business, product development and commercialization efforts. Reliance on Third-Party Suppliers and Service Providers For some of our raw materials, we rely on a single source of supply and there are limited supplies of the raw materials. If we were to experience an unexpected loss of supply of or if any supplier was unable to meet our demand for any of our products and product candidates, including for example if VYVGART is approved for additional indications, we could experience delays in our research or planned clinical trials or risk shortages in commercial supply which could materially impact our revenue potential. These issues could be made worse during a pandemic or due to geopolitical events, including trade disputes or economic sanctions enacted as a result of international conflict. Additionally, certain of the raw materials required in the manufacture and the formulation of our products and product candidates may be derived from biological sources, including mammalian tissues, bovine serum and human serum albumin. There are certain European regulatory restrictions on using these biological source materials including rigorous testing requirements, which could limit or delay production. If there are changes in the regulation ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Dependence on Third Parties | 124 |
| requirements that our suppliers are unable to meet, our clinical development or commercial activities may be delayed or interrupted. We may not be able to engage a back-up or alternative supplier or service provider in a timely manner or at all if any of these third parties were to cease or interrupt production or otherwise fail to supply these materials, products, or services to us for any reasons, including due to regulatory requirements or actions (including recalls), adverse financial developments at or affecting the supplier, failure by the supplier to comply with cGMPs, contamination, business interruptions, or labor shortages or disputes. Interruptions in the supply of these materials, products or services may also result from international conflict, trade disputes or economic sanctions enacted by, or imposed on, the U.S., the UK, the EU or any other country or region. Reliance on Third-Party Manufacturing We rely on and expect to continue to rely on CMOs. We also rely on certain third parties to perform filling, finishing, distribution, laboratory testing and other services related to the manufacture of our products and product candidates. Although we do not control the manufacturing process at our CMOs and are completely dependent on them for the production of our products and product candidates in accordance with relevant regulations (such as cGMPs), we are responsible for ensuring that our products comply with regulatory requirements. If our CMOs cannot successfully manufacture material that conforms to our specifications and the strict regulatory requirements of the Relevant Regulatory Authorities or other comparable regulatory authorities, our business could be adversely affected in a number of ways, including an inability to initiate or continue clinical trials of product candidates under development, delay in submitting regulatory applications, or receiving regulatory approvals for product candidates, including new indications, subjecting third-party manufacturing facilities to additional inspections by regulatory authorities, requirements to cease distribution or to recall batches of our products or product candidates and an inability to meet commercial demands for our marketed products. We contract with Lonza based in Slough, UK, Portsmouth, U.S., Singapore and Visp, Switzerland and Fujifilm based in Denmark for activities relating to the development of cell banks, development of our manufacturing processes and the manufacturing of drug substance, and use additional contract manufacturers to fill, test, label, package, store and distribute our (investigational) drug products. Our products and product candidates are biologics and require multiple processing steps that are more difficult than those required for most small molecule chemical pharmaceuticals. Problems with these manufacturing processes, such as capacity issues, or even minor deviations from the normal process or from the materials used in the manufacturing process, which may not be detectable by us in a timely manner, could lead to manufacturing failures or product defects, resulting in lot failures, product recalls, product liability claims and insufficient inventory. We face risks inherent in relying on limited CMOs, as any failure in their ability to successfully manufacture our products or product candidates as described above or any disruption, such as a fire, pandemic, natural hazards or vandalism at the CMO could significantly interrupt our manufacturing capability. Alternative production plans in place or disaster-recovery facilities available to us may not be sufficient. In case of a disruption, we may have to establish additional alternative manufacturing sources. This would require substantial investment on our part, which we may not be able to obtain on commercially acceptable terms or at all. Additionally, we may experience significant manufacturing delays as we build or locate replacement facilities and seek and obtain necessary regulatory approvals. If this occurs, we will be unable to satisfy manufacturing needs on a timely basis, if at all. Also, operating any new facilities may be more expensive than operating at our current facilities. Further, business interruption insurance may not adequately compensate us for any losses that may occur, and ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Dependence on Third Parties | 125 |
| 我們將不得不承擔任何中斷的額外成本。出於這些原因,製造設施的重大中斷事件可能會產生嚴重後果,包括 將我們的財務穩定性置於風險之中。 我們財務報告的準確性和時間部分取決於從第三方合作伙伴那裏收到的信息,而我們無法控制這些信息。 我們已經就某些候選產品與第三方合作,並計劃繼續合作,其中包括 總代理商和許可合作伙伴。作為這些 協作的一部分,我們的協作合作伙伴負責向我們提供有關特定項目的財務 信息,包括花費的資金、產生的負債和預期的 未來成本,我們自己的財務報告依賴於這些信息。如果我們的協作合作伙伴未能在商定的時間範圍內向我們提供必要的財務信息,或者 如果此類財務信息被證明不準確,將對我們自己財務報告的時間和準確性產生不利影響。我們財務報告中的任何錯誤都可能導致 投資者對我們的財務報告失去信心。這進而可能導致聲譽 損害或影響我們獲得任何未來融資的能力和條款,這可能會損害我們的業務。 我們和我們的第三方製造商和供應商可能面臨與環境合規或補救活動相關的責任、罰款、處罰或其他制裁和鉅額費用。 我們的第三方製造商和供應商的運營,包括研究、開發、測試和製造活動,受到許多環境、健康和 安全法律法規的約束。除其他事項外,這些法律和法規規定了危險材料和生物材料的受控使用、處理、釋放和處置以及登記、實驗室程序和接觸病原體的情況。我們無法控制製造商或供應商遵守環境、健康和安全法律法規的情況。如果我們或他們未能遵守此類法律和法規,我們可能會受到責任、罰款、處罰或其他制裁,並且 將產生大量費用來遵守或補救這些活動。 我們面臨當前和歷史活動固有的環境責任風險,包括與釋放或接觸危險或生物材料有關的責任。 環境、健康和安全法律法規正變得更加嚴格。我們可能需要 產生與未來環境合規或補救活動相關的鉅額費用 ,在這種情況下,我們的生產和開發工作可能會中斷或延遲,我們的財務狀況和運營結果可能會受到重大不利影響。 與Argenx 工商 我們的員工可能從事不當行為或其他不當活動,包括違反監管標準和要求, 或違反內幕交易,這可能會嚴重損害我們的業務。 我們面臨員工欺詐或其他不當行為的風險。員工的不當行為 可能包括故意不遵守政府法規、遵守美國和其他國家的醫療欺詐和濫用以及反回扣法律法規 2.7Argenx Capital 股票財務 審查聲明 財務非財務 信息 ar Gr g oup enx Factors Risk Go Corporate vernance 2023年商業和工業年度報告|126 |
| 未向我們準確報告財務信息或數據,或未向我們披露未經授權的活動。特別是,醫療保健行業的銷售、營銷和業務安排受到旨在防止欺詐、不當行為、回扣、自我交易和其他濫用行為的廣泛法律法規的約束。這些法律法規可能限制或禁止廣泛的定價、折扣、營銷和促銷、銷售佣金、客户激勵計劃和其他業務安排。員工不當行為還可能涉及對重要信息的不當使用,包括基於臨牀試驗過程中獲得的信息進行的不當交易,這可能會導致監管制裁 並嚴重損害我們的聲譽。我們維護全球合規計劃,並繼續 專注於該計劃的發展和增強。我們的計劃包括風險 評估和監控、培養鼓勵員工和第三方提出誠意問題或關切的文化,以及為審查和 補救指控和確定潛在關切而定義的流程和系統。然而,並非總是可以識別和阻止員工的不當行為,我們為檢測和防止此類行為而採取的預防措施可能無法有效控制未知或未管理的風險或損失 或保護我們免受因未能遵守這些法律或法規而引發的政府調查或其他行動或訴訟。如果對我們提起任何此類訴訟,而我們未能成功為自己辯護或維護自己的權利,這些 行動可能會對我們的業務和運營結果產生重大影響,包括 施加鉅額罰款或其他制裁。 我們可能面臨代價高昂的破壞性責任索賠。 我們面臨潛在的產品責任和專業賠償風險,這些風險是 藥品的研究、開發、製造、營銷和使用以及人類治療產品的營銷所固有的。我們和我們的合作者目前和將來在臨牀試驗中使用的產品和候選產品,以及銷售任何經批准的產品,可能會進一步使我們面臨責任索賠。如果我們的任何產品或 候選產品在臨牀試驗期間或在 候選產品批准後導致不良副作用,我們可能會承擔重大責任。這些索賠可能是由使用該產品的患者、醫療保健提供者、製藥公司、醫生、付款人、照顧者、投資者、員工、政府機構或我們的 合作者或其他銷售此類產品的人提出的。醫生和患者可能不遵守確定已知潛在不良反應的任何警告,以及不應使用我們的候選產品的患者 。針對我們的任何索賠,無論其是非曲直,都可能難以抗辯且成本高昂,並可能對我們的產品和候選產品的市場或我們的產品和候選產品的任何商業化前景產生重大不利影響。任何此類索賠,無論其是非曲直,都可能對我們的聲譽以及醫生和患者對我們產品的信任造成不利影響。如果對當前歐盟立法程序處於後期階段的當前歐盟立法進行有利於原告的改革,歐盟的產品責任風險未來可能會增加 。 無論是非曲直或最終結果訴訟或責任索賠可能導致: ·由於公眾的負面看法,對我們產品的需求減少; ·損害我們的聲譽; ·臨牀試驗參與者退出或難以招募新的臨牀試驗參與者; ·監管機構發起調查; ·辯護或解決相關訴訟的費用; ·轉移管理層的時間和我們的資源; ·對臨牀試驗參與者或patients; ar Gr g oup enx Factors Risk Go Corporate vernance資本的鉅額金錢獎勵 股票財務 審查報表 財務非財務 信息 Argenx 2023年工商年報|127 |
| · product recalls, withdrawals or labeling, marketing or promotional restrictions; · loss of revenues from product sales; and · the inability to successfully commercialize VYVGART and any of our other product candidates, if approved. Although we maintain product liability insurance, we may not be able to maintain insurance coverage at a reasonable cost or to obtain adequate insurance coverage to satisfy any liability that may arise. Product liability claims could delay or prevent completion of our clinical development programs. In addition, claims made by patients, healthcare professionals or others might not be fully covered by product liability insurance and could result in investigations of the safety of our products or product candidates or may result in recalls. If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, our assets may not be sufficient to cover such claims and our business, financial condition and results of operations would be adversely affected. We may engage in strategic transactions, including acquisitions, collaborations, licenses or investments in other companies or technologies, and we may not realize the benefits of such transactions. We may enter into strategic transactions, including acquisitions, collaborations, licenses or investments for or in other companies or technologies that complement or augment our existing business and facilitate our access to new products, research projects or geographical areas. However, we may not be able to identify appropriate targets or enter into such transactions under satisfactory conditions. In addition, we may need additional funding to finance these transactions including through issuances of public or private equity or convertible debt securities, which could be dilutive to our shareholders and ADS holders. Integrating any newly acquired companies, business, technologies or products could be expensive, time-consuming, and may never be successful. Integration efforts often take a significant amount of time, place a significant strain on managerial, operational and financial resources, result in loss of key personnel and could prove to be more difficult or expensive than we predict. The diversion of our management’s attention and any delay or difficulties encountered in connection with any future transactions we may consummate could result in the disruption of our ongoing business or inconsistencies in standards and controls that could negatively affect our ability to maintain third-party relationships. We cannot assure that we will achieve the expected synergies to justify any such transaction, which could have a material adverse effect on our business, financial condition, results of operations and future growth prospects and our investors’ ability to realize on their investment. Our business and operations could suffer in the event of system failures or unauthorized or inappropriate use of or access to our systems. We are increasingly dependent on our information technology systems and infrastructure for our business. We collect, store and transmit sensitive information including intellectual property, proprietary business information, including highly sensitive clinical trial data, and personal data in connection with business operations. The secure maintenance of this information is critical to our operations and business strategy. Some of this information could be an attractive target of criminal attack or unauthorized access and use by third parties with a wide range of motives and expertise, including organized criminal groups, “hacktivists,” patient groups, disgruntled current or former employees and others. Cyber-attacks are of ever-increasing levels of sophistication, and despite our security measures, our information technology and infrastructure may be vulnerable to such attacks or may be breached, including due to employee error or malfeasance. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Business and Industry | 128 |
| The pervasiveness of cybersecurity incidents in general and the risks of cyber-crime are complex and continue to evolve. Although we are making significant efforts to maintain the security and integrity of our information systems and are exploring various measures to manage the risk of a security breach or disruption, there can be no assurance that our security efforts and measures will be effective or that attempted security breaches or disruptions would not be successful or damaging. Despite the implementation of security measures, our internal computer systems and those of our contractors and consultants are vulnerable to damage or interruption from computer viruses, unauthorized or inappropriate access or use, natural disasters, pandemics, terrorism, war (including the ongoing conflict in Ukraine and the ongoing conflict in Israel and the Gaza Strip), and telecommunication and electrical failures. Such events could cause interruption of our operations. For example, the loss of pre-clinical trial data or data from completed or ongoing clinical trials for our product candidates could result in delays in our regulatory filings and development efforts, as well as delays in the commercialization of our products, and significantly increase our costs. To the extent that any disruption, security breach or unauthorized or inappropriate use or access to our systems were to result in a loss of or damage to our data, or inappropriate disclosure of confidential or proprietary information, including but not limited to patient, employee or vendor information, we could incur notification obligations to affected individuals and government agencies, liability, including potential lawsuits from patients, collaborators, employees, stockholders or other third parties and liability under foreign, federal and state laws that protect the privacy and security of personal data, and the development and potential commercialization of our product candidates could be delayed. We are highly dependent on public perception of our products. We are highly dependent upon consumer perceptions of the safety and quality of our products. We could be adversely affected if we, or any of our collaborators, are subject to negative publicity or if any of our products or any similar products distributed by other companies prove to be, or are asserted to be, harmful to patients, or for example, be deemed cruel to animals. Because of our dependence upon consumer perception, any adverse publicity associated with illness or other adverse effects resulting from patients’ use or misuse of our products or any similar products distributed by other companies could have a material adverse impact on our business, prospects, financial condition and results of operations. Risk Factors Related to argenx’s Intellectual Property Failure to adequately enforce or protect our intellectual property rights in products, product candidates and platform technologies could adversely affect our ability to maximize the value for patients in our marketed products and product candidates. Our commercial success depends in part on obtaining and maintaining patents and other forms of intellectual property rights for our products, product candidates and platform technologies. Failure to protect or to obtain, maintain or extend adequate patent and other intellectual property rights, which may be challenging and costly, could adversely affect our ability to develop and market our products and product candidates and erode or negate any competitive advantage we may have. We cannot be certain that patents will be issued or granted with respect to applications that are currently pending. The scope of patent protection that the European Patent Office and the USPTO will grant with respect to the antibodies in our product pipeline is uncertain and may vary by jurisdiction. It is possible that the European Patent Office and the USPTO will not allow 2.8 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 129 |
| broad antibody claims that cover antibodies closely related to our products and product candidates as well as the specific antibody. As a result, upon receipt of EMA or FDA approval, competitors may be free to market molecules almost identical to ours if those competitors elected to engage and invest in a full clinical development program to establish the safety and efficacy of their molecule and secure that approval. If that would happen, then there is a risk of decreasing our market potential. We and our current or future licensors, licensees or collaboration partners may not be able to prepare, file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner. Further, the issuance, scope, validity, enforceability and commercial value of our and our current or future licensors’, licensees’ or collaboration partners’ patent rights are highly uncertain. Moreover, in some circumstances, we may need to rely on patent procurement activities of our licensors, licensees or collaboration partners or obtain additional costly licenses. Such parties may not fully comply with applicable patent rules or disagree with us as to the prosecution, maintenance or enforcement of any patent rights. Even if patents do issue and such patents cover our products and product candidates, third parties may initiate proceedings challenging the validity, enforceability or scope of such patents, which may result in the patent claims being narrowed or invalidated. Our and our licensors’, licensees’ or collaboration partners’ patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless and until a patent issues from such applications, and then only to the extent the issued claims cover the technology. Furthermore, because patent applications are confidential for a period of time after filing, and some remain so until issued, we cannot be certain that we or our licensors were the first to file any patent application related to a product and product candidate. Even where we have a valid and enforceable patent, we may not be able to exclude others from practicing our invention where the other party can show that they used the invention in commerce before our filing date, or if the other party is able to obtain a compulsory license. In addition, after expiry of our regulatory exclusivities, we may not have a patent position to enforce if a biosimilar presents a ‘skinny label’ and introduces a product into commerce for unpatented uses, doses, and other important patient innovations described in our product labels. Any of the aforementioned situations could cause harm to our ability to protect our intellectual property, which in turn would allow competitors to market comparable products which could materially adversely affect our competitive position and as such our business, financial condition and results of operation. We enjoy only limited geographical protection with respect to certain patents and may face difficulties in certain jurisdictions. We often file our first patent application (i.e., priority filing) at the UK Intellectual Property Office, the European Patent Office or the USPTO. International applications under the Patent Cooperation Treaty are usually filed within 12 months after the priority filing. We have so far not filed for patent protection in all national and regional jurisdictions where such protection may be available. If we fail to timely file a patent application in any such country or major market, we may be precluded from doing so at a later date. In addition, the grant proceeding of each national/regional patent may lead to situations in which applications might in some jurisdictions be refused by the relevant patent offices, while granted by others. Furthermore, competitors may use our and our licensors’ or collaboration partners’ technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we and our licensors or collaboration partners have patent protection, but enforcement is not as strong as that in the U.S., UK and the EU. Finally, some countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties, and other countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 130 |
| Issued patents could be found invalid or unenforceable if challenged in the applicable patent office or court. Once granted, patents may remain open to invalidity challenges after allowance or grant, where third parties can raise objections against such granted patent. In the course of such proceedings, the patent owner may be compelled to limit the scope of the allowed or granted claims thus challenged or may lose the allowed or granted claims altogether. To protect our competitive position, we may from time to time need to resort to adversarial proceedings in order to enforce or defend any intellectual property rights owned by or licensed to us, or to determine or challenge the scope or validity of intellectual property rights of third parties. Enforcement of intellectual property rights is difficult, unpredictable and expensive, and many of our or our licensors’ or collaboration partners’ adversaries in these proceedings may have the ability to dedicate substantially greater resources to prosecuting these legal actions than we or our licensors or collaboration partners can. In addition, adversarial proceedings involving our patents carries the risk that one or more of our patents will be held invalid or held unenforceable. Such an adverse ruling could allow third parties to commercialize our products immediately after the expiration of our regulatory protection or use our platform technologies, and then compete directly with us, without payment to us. We may be subject to claims challenging the inventorship or ownership of our intellectual property or be required to make additional payments to secure intellectual property from collaborators. Many of our consultants and employees, including our senior management, were previously employed at other biotechnology or pharmaceutical companies, including our competitors or potential competitors. Some of these consultants and employees executed proprietary rights, non-disclosure and non-competition agreements in connection with such previous employment. Although we try to ensure that our consultants and employees do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or these consultants and employees have used or disclosed confidential information or intellectual property of any such consultant’s or employee’s former employer or have breached their non-competition agreement. Additionally, many of our collaborators do not commit to assigning all intellectual property arising out of the collaboration to us and, instead, grant us options to acquire intellectual property or commit to making such intellectual property available to us at a fair price. As such, we or our licensors may have inventorship disputes arise from conflicting obligations of employees, consultants or others who are involved in developing our products and product candidates. In addition, while it is our policy to require our employees and contractors who may be involved in the development of intellectual property to execute agreements assigning such intellectual property to us, we may be unsuccessful in executing such an agreement with such party. Our and their assignment agreements may not be self-executing or may be breached and we may be forced to bring claims against third parties or defend claims they may bring against us to determine the ownership of what we regard as our intellectual property. There is no guarantee we will be successful in defending such claims, which would result in us paying monetary damages, or lose valuable personnel or intellectual property rights. Third-party intellectual property rights could adversely affect our ability to commercialize our products and product candidates. Our competitive position may suffer if third-party intellectual property rights cover our products or product candidates or our manufacture or uses relevant to our development plans. In such cases, we may not be in a position to develop or commercialize products or product candidates unless we secure a license, design around, or successfully pursue costly ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 131 |
| and time-consuming proceedings to nullify or invalidate the third-party intellectual property right concerned or enter into a license agreement with the intellectual property right holder. If we were to challenge the validity of any issued U.S. patent in court, we would need to overcome a statutory presumption of validity that attaches to every U.S. patent. In the event that a patent is successfully asserted against us such that the patent is found to be valid and enforceable and infringed by our product, unless we obtain a license to such a patent, we could be prevented from continuing to develop or commercialize our product. Similarly, other companies have filed patent applications or have patents on the targets for certain of our products or their uses. There can be no assurance any such patents will not be asserted against us or that we will not need to seek licenses from such third parties. It is also possible that we are unaware of relevant patents or applications or of relevant scientific discoveries. In general, patent applications in the U.S. and elsewhere are published approximately 18 months after the earliest filing from which priority is claimed, with such earliest filing date being commonly referred to as the priority date. Additionally, publications of discoveries in scientific literature often lag behind the actual discoveries. Therefore, patent applications covering our products, product candidates or platform technology could have been filed by others and relevant discoveries may have been made without our knowledge. Additionally, pending patent applications which have been published can, subject to certain limitations, be later amended in a manner that could cover our products or platform technologies. Third-party intellectual property right holders, including our competitors, may actively bring infringement claims against us that we may not be able to successfully settle or otherwise resolve. If we fail in any such dispute, we or our licensees may be prohibited from commercializing any of our products and product candidates that are held to be infringing for the remaining term of any valid and enforceable patents. We might, if possible, also be forced to redesign products and product candidates so that we no longer infringe the third-party intellectual property rights. We may be required to seek a license to any such technology that we are found to infringe, which license may not be available on commercially reasonable terms, or at all. Even if we or our licensors or collaboration partners obtain a license, it may be non-exclusive, thereby giving our competitors access to the same technologies licensed to us or our licensors or collaboration partners. In addition, if the breadth or strength of protection provided by our or our licensors’ or collaboration partners’ patents and patent applications is threatened, it could dissuade companies from collaborating with us to license, develop or commercialize current products and product candidates. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. We may not be successful in obtaining or maintaining necessary rights to our products and product candidates through acquisitions and in-licenses. We may be unable to acquire or in-license third-party intellectual property rights that we identify as an appropriate strategic fit for our Company and necessary for our product candidates and technology. A number of more established companies with greater resources may pursue strategies to license or acquire third-party intellectual property rights that we may consider attractive. We sometimes collaborate with U.S. and non-U.S. academic institutions to accelerate our preclinical research or development. Typically, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 132 |
| 協作。無論如何,我們可能無法在 指定的時間範圍內或在我們可接受的條款下談判許可證,在這種情況下,機構 可能會將知識產權提供給其他方,這可能會阻止我們 追求適用的候選產品或計劃的能力。 此外,將我們視為競爭對手的公司可能不願將許可權轉讓或 轉讓給我們。我們也可能無法許可或獲取第三方知識產權 ,其條款將使我們的 投資獲得適當回報,在這種情況下,我們可能不得不放棄該候選產品或 計劃的開發。 現有的許可協議規定了各種開發、付款和其他義務。如果 我們未能履行這些協議下的義務,許可方可能有 權利終止許可,這可能導致我們無法開發、製造、營銷和銷售許可技術涵蓋的產品。此外,我們可能會 產生鉅額成本和/或中斷我們的業務,並分散我們管理層的注意力 針對許可方指控的任何違規行為進行辯護。我們現有的幾個許可證 協議是來自第三方的子許可證,這些第三方不是所涉知識產權的原始許可方。如果許可方未能履行其在這些上游許可協議下的義務,則原始第三方許可方可能有權終止 原始許可,這可能會終止再許可,如果我們不能以合理條款與相關權利的所有者 獲得我們自己的直接許可,則我們將失去對適用知識產權的權利。 此外,如果圍繞我們已許可的知識產權或我們相關的 義務的爭議阻礙或削弱了我們以可接受的條款維持當前許可安排的能力,我們可能無法成功開發受影響的產品和候選產品並將其商業化。 如果我們的商標和商品名稱沒有得到充分保護,我們可能無法在我們感興趣的市場上建立知名度。 我們的註冊或未註冊商標或商品名稱可能會受到質疑、侵權、規避或宣佈為通用商標,或被確定為侵犯了其他商標。第三方 可能反對或試圖取消我們的商標申請或商標,或以其他方式挑戰我們對商標的使用。如果我們的商標被成功地 挑戰,我們可能無法使用這些商標在這些 國家/地區營銷我們的產品,並可能被迫重新命名我們的產品,這可能會導致失去品牌 認知度,並可能需要我們投入資源來廣告和營銷新的 品牌。如果我們試圖強制執行我們的商標並主張商標侵權索賠, 法院可能會裁定我們主張的商標無效或不可強制執行,或者我們主張商標侵權的 方對相關商標擁有更高的權利。從長遠來看,如果我們無法建立知名度,我們可能 就無法有效競爭。如果其他實體在不同的 司法管轄區使用與我們的商標類似的商標,或者對我們的商標擁有優先權利,可能會干擾我們在世界各地使用我們當前的商標。 我們可能無法獲得《哈奇-瓦克斯曼法》和類似的非美國法律的保護,以延長我們的每個產品和候選產品的專利期。 根據FDA對我們的候選產品進行上市批准的時間、期限和條件,我們的一項或多項美國專利可能有資格根據《哈奇-瓦克斯曼法案》以及歐盟和亞太地區的類似立法獲得有限專利期的延長 。《哈奇-瓦克斯曼法》允許覆蓋經批准產品的專利最多延長五年,作為對失去有效專利期的during ar Gr g oup enx Factors Risk Go Corporate vernance資本的補償 股票財務 審查聲明 財務非財務 信息 argenx 2023年知識產權年度報告|133 |
| product development and the FDA regulatory review process. The patent term extension cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, and only one patent applicable to an approved drug may be extended. However, we may not receive an extension if we fail to apply within applicable deadlines or prior to expiration of relevant patents or otherwise fail to satisfy applicable requirements. Moreover, the length of the extension could be less than we request. If we are unable to obtain patent term extension or the term of any such extension is less than we request, the period during which we can enforce our patent rights for that product will be shortened and our competitors may obtain approval to market competing products sooner than we expect. Changes in patent laws or patent jurisprudence could diminish the value of patents in general, thereby impairing our ability to protect our products. Changes in patent law and regulations in the various countries or jurisdictions or changes in the governmental bodies that enforce them or changes in how the relevant governmental authority enforces them may weaken our ability to obtain new patents or to enforce patents that we have licensed or that we may obtain in the future. We cannot predict future changes in the interpretation of patent laws or changes to patent laws that might be enacted into law by U.S. and foreign legislative bodies. For example, the U.S. Supreme Court has ruled on several patent cases in recent years, either narrowing the scope of patent protection available in certain circumstances or weakening the rights of patent owners in certain situations. Relatedly, U.S. congressional representatives have introduced multiple draft bills this year that, if passed, may have a significant impact on U.S. patent laws. Such changes by the U.S. Congress, U.S. courts, USPTO, and the relevant law-making bodies in other countries may materially affect our patents or patent applications and our ability to obtain additional patent protection in the future. We may be unable to protect the confidentiality of our trade secrets and know-how. In addition to patent protection, we rely on trade secret protection for our proprietary information, including, for example, certain aspects of our llama immunization and antibody affinity maturation approaches. However, trade secrets are difficult to protect, and we have limited control over the protection of trade secrets used by our numerous licensors, collaborators and suppliers. We require our employees, consultants, advisors and potential collaborators to enter into confidentiality agreements. Moreover, we put in place appropriate procedures to identify confidential material and restrict access to documentation. However, current or former employees, consultants, advisors and potential collaborators may unintentionally or willfully disclose our confidential information to competitors despite these procedures or in violation of our confidentiality agreements. In addition, the need to share trade secrets and other confidential information increases the risk that such trade secrets become known to our competitors or inadvertently incorporated into the technology of others. Any disclosure, either intentional or unintentional, or misappropriation by third parties (such as through a cybersecurity breach) of our trade secrets or proprietary information could enable competitors to duplicate or surpass our technological achievements. Enforcing a claim that a third party illegally obtained and is using trade secrets is expensive, time-consuming and the outcome is unpredictable, and the enforceability of confidentiality agreements may vary from jurisdiction to jurisdiction. Moreover, if any of our trade secrets were to be lawfully obtained or independently developed by a competitor or other third party, we would have no right to prevent them from using that technology or information to compete with us. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intellectual Property | 134 |
| Risk Factors Related to argenx’s Organization and Operations Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel. As a global organization in a highly competitive and specialized industry, our success depends upon the continued contributions of our key management, scientific, medical and technical personnel, many of whom have been instrumental for us and have substantial experience with our product and related technologies. These key management individuals include the members of our Board of Directors and senior management team. Difficulties in recruiting or the loss of key managers, scientific, medical or technical personnel could delay our research and development activities. In addition, it may be difficult to attract and retain highly qualified management, scientific and medical personnel, particularly if we expand into fields that will require additional skills. As a Dutch company listed on Euronext Brussels in addition to Nasdaq, our remuneration practices and policies may be limited by local governance rules or shareholder guidance for EU companies. Such limitations may make it more difficult to successfully compete for key talent in a number of markets that have differing remuneration practices and policies as we are bound by more restrictive remuneration practices than our competitors. For example, the Dutch Corporate Governance Code 2022 (DCGC) places certain limitations on the ability to grant equity incentives to non-executive directors, while Belgian law requires non-executive directors to receive part of their remuneration in the forms of shares, but not stock options. The DCGC also places limitations on amount of severance payment permitted in the event of dismissal. In addition, the U.S. has proposed legislation that imposes restrictions on our ability to prevent departing employees from competing with us following their departure. If finalized, such legislation could also adversely affect our ability to retain employees who may go to competitors with more resources than us and who are not bound by similar remuneration policies. Many other biotechnology and pharmaceutical companies and academic institutions that we compete against for qualified personnel have greater financial and other resources, different risk profiles and a longer history in the industry than we do. Additionally, an inflationary environment, combined with the tight labor market for the recruitment and retention of skilled workers, could make it more costly for us to attract or retain employees. In order to meet the compensation expectations of our prospective and current employees due to inflationary factors, we may be required to increase our operating costs. Therefore, we might not be able to attract or retain these key persons on conditions that are economically acceptable. Global geo- and socio-political threats and macro-economic uncertainty and other unforeseen political crises could materially and adversely affect our business and financial performance. Many geo- and socio-political threats and macro-economic uncertainties are outside of our control, including general economic and market conditions, consumer and commercial credit availability, inflation, interest rates, unemployment, consumer debt levels, political crises, such as terrorist attacks, war and other political instability, economic sanctions, outbound investment restrictions and other challenges affecting the global economy, including the Russia-Ukraine and the Israel-Hamas conflicts, disruptions in supply chains, and changes in trade agreements which could adversely affect consumer confidence and disposable income levels, increase difficulty in forecasting our financial results and have other impacts on our 2.9 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Organization and Operations | 135 |
| 業務和財務業績。這種地緣和社會政治威脅還可能導致股市整體波動,導致我們的股票出現與我們的業務和財務業績無關的極端價格和成交量波動。例如,2023年,我們 通過我們的合作伙伴Medison獲得了VYVGART在以色列的批准。目前還無法預測或確定持續的以色列-哈馬斯衝突對我們的業務和財務業績的最終後果。 由於我們的國際業務,以及我們在大量 司法管轄區進行臨牀試驗的事實,全球衝突的爆發,如俄羅斯和烏克蘭之間持續的衝突以及以色列-哈馬斯的持續衝突,可能會對我們在受影響地區進行或完成臨牀試驗的能力產生負面影響,這可能會對我們的業務和財務業績產生不利影響。2023年1月17日,美國財政部外國資產管制辦公室頒發了通用許可證6C,以取代通用許可證6B。通用許可證6C授權“臨牀試驗和其他醫學研究活動” ,否則將被美國針對俄羅斯的制裁所禁止,而通用許可證6C 沒有到期日。在進行了與俄羅斯和烏克蘭衝突有關的風險評估後,我們增加了目標註冊人數,這將光伏和光伏的SC efgartigimod的預期TOPLINE數據 推遲到2023年下半年。此外,俄羅斯和烏克蘭之間的衝突以及美國、英國和歐盟等對俄羅斯實施的制裁可能會擾亂: ·可能無法安全前往臨牀試驗地點或可能因多種原因被迫退出的合格患者的招募和登記; ·關閉或摧毀臨牀地點或治療設施; ·補償居住在受制裁國家的患者或工作人員的能力; ·我們產品或供應鏈的製造流程,這可能會增加原材料成本和生產成本; ·將必要的材料、產品或服務運輸、交付、供應和收集到臨牀試驗地點或交付給第三方中心實驗室進行分析的能力; ·從臨牀試驗地點收集數據並確保收集的任何數據的完整性的能力 ; ·我們的數據中心或關鍵業務或信息技術系統遭到破壞或中斷 ;或 ·由於不完整 或監管機構無法完全審核的事實,無法提交在俄羅斯或烏克蘭網站收集的數據。 截至目前,除上述和本年度報告中的其他地方外,我們沒有 跡象表明,俄羅斯和烏克蘭之間的衝突以及對俄羅斯實施的相應制裁將顯著阻礙我們在受影響地區進行的臨牀開發活動,或與我們正在等待或預期的審批請求相關的監管活動 。此外,我們在俄羅斯不產生收入,我們從俄羅斯和烏克蘭的利益相關者和團隊成員那裏收集更多定期 反饋。然而,我們 也在俄羅斯和烏克蘭的一些鄰國進行開發活動 ,如果衝突進一步升級並影響鄰國,可能會影響我們在這些國家的發展活動。 美國與大陸中國關係的變化,包括關税、出口管制、制裁和 其他法規可能會對我們與再鼎醫藥在大中國地區的合作產生不利影響。美國政府已經並將繼續採取措施處理將影響與美國或大陸有聯繫的公司的美中關係 中國 中國,包括對某些在大陸生產的產品徵收關税 中國,對在大陸的個人和實體實施某些制裁,和 發佈聲明,加強對擁有大量內地China-ar Gr g oup enx Factors Risk Go Corporate vernance資本的公司的審查 股票財務 審查報表 財務非財務 信息 argenx 2023年組織和運營年報|136 |
| based operations. The scope of the impact of any such actions on the pharmaceutical industry or argenx remains unknown. Through argenx’s collaboration with Zai Lab, we have an interest in business operations in Greater China. Any unfavorable government policies on international trade, including tariffs, export controls, and/or increased scrutiny on companies with significant Mainland China-based operations, may impact the development and commercialization of products that contain argenx-licensed material. Any new legislation, executive orders, tariffs, export controls, and/or other regulations that may be implemented, the renegotiation of existing trade agreements, and any retaliatory actions taken by the U.S. or Chinese governments due to the U.S.- Mainland China tensions could have an adverse effect on our business, including the development and commercialization of products containing argenx-licensed material. We face risks related to natural disasters and public health issues, that could negatively affect our business and financial condition. Our business could be adversely impacted by the effects of catastrophic global events including natural disasters such as an earthquake, fire, hurricane, tornado, flood or significant power outage and pandemics, such as the COVID-19 pandemic. For example, the manufacturing of all of our products and product candidates requires using cells which are stored in a cell bank. We have one master cell bank for each product manufactured in accordance with cGMPs. However, it is possible that we could lose multiple cell banks and have our manufacturing significantly impacted by the need to replace these cell banks, which could materially adversely affect our business, prospects, financial condition and results of operations. Public health issues could also negatively affect our business and financial condition. We operate and conduct our clinical trials globally. We cannot presently predict the scope and severity of any potential future business shutdowns or disruptions as a result of public health issues. If we or any of the third parties with whom we engage, including the suppliers, contract manufacturers, clinical trial sites, regulators and other third parties, were to experience shutdowns, quarantines, or other business disruptions due to natural disasters or global public health issues, it may impair our or our third-party partners’ ability to initiate clinical trials and recruit and retain patients, particularly if quarantine or travel restrictions impede healthcare provider or patient movement, impact the usability of the data due to treatment interruptions and require protocol amendments. In addition, regulatory authorities may restrict their operations or be delayed in their operations during a pandemic, the outbreak of new variants or other public health issues, including further to travel restrictions which could adversely affect our ability to obtain regulatory approval for and to commercialize our products and product candidates and have a material adverse effect on our business and financial results. We may encounter difficulties efficiently managing our growth and our increasing development, regulatory and sales and marketing capabilities, which could disrupt our operations. We have grown significantly in the number of employees and scope of operations over recent years and expect to experience significant growth in the number of our employees and the scope of our operations also in the near future, particularly in the areas of drug research, drug development, regulatory affairs and sales and marketing. To manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities and continue to recruit and train additional qualified personnel. In particular, we must efficiently leverage our own sales and marketing capabilities in order to launch or market our products candidates effectively. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Organization and Operations | 137 |
| Due to our limited financial resources and the limited experience of our management team in managing a company with such anticipated growth, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. Our limited financial, manufacturing and management resources, could cause us to forgo or delay the pursuit of opportunities with potential product candidates that later prove to have greater market potential, fail to capitalize on viable commercial products or profitable market opportunities or relinquish rights to such product candidates through collaborations, licensing or royalty arrangements in circumstances where it would have been more advantageous for us to retain sole development and commercialization rights. Any inability to manage growth could delay the execution of our strategic objectives or disrupt our operations, which in turn could materially harm our business and prospects. We have benefitted from certain research and development incentives. These could be impacted by changes in law (or interpretation), changes of fact (such as a change in ownership), or challenge by tax authorities. As a company active in research and development, we have benefited from certain research and development tax incentives including tax credits and a payroll withholding tax exemption. We also expect to benefit from the Belgian innovation income deduction. The relevant tax authorities may challenge our eligibility for, or our calculation of, such tax incentives and, should such a challenge be successful, we may be liable for additional taxes, and penalties and interest related thereto, which could have an impact on our results of operations and future cash flows. In case of a change of control, we could be exposed to the risk of losing any unused tax credit and innovation income deduction. Furthermore, if any legislator decides to eliminate, or change the conditions for claiming such tax incentives, or reduce the scope or the rate of such incentives, any of which it could decide to do at any time, our results of operations could be adversely affected. We are exposed globally to unanticipated changes in tax laws and regulations, adjustments to our tax provisions, exposure to additional tax liabilities, or forfeiture of our tax assets. The determination of our provision for income taxes and other tax liabilities requires judgment, including the adoption of certain accounting policies and our determination of whether our deferred tax assets are, and will remain, fully available in future periods. We cannot guarantee that our interpretation of applicable tax laws or our structure will not be questioned by the relevant tax authorities, or that the relevant tax laws and regulations, or the interpretation thereof, including through tax rulings, by the relevant tax authorities, will not be subject to change. Any adverse outcome of such a review or change in law may lead to adjustments in the amounts recorded in our financial statements and could have a materially adverse effect on our operating results and financial condition. Dealings between group companies are subject to transfer pricing regulations, which may be subject to change and could have a material impact. Compliance with these laws and regulations will be more challenging as we expand our international operations, including in connection with potential approvals of our products and product candidates in Europe, Japan, the U.S. and elsewhere. Our effective tax rates could be adversely affected, now or in the future, by changes in tax laws, treaties and regulations or the interpretation thereof by the relevant tax authorities in countries where we have material operations, including changes to the Belgian innovation income deduction, to the corporate income tax base, or to other tax incentives and the implementation of new tax incentives. A successful challenge to our qualifications for and application of these tax incentives by the tax authorities in Belgium or other country where we have material operations would have a significant impact on our effective tax rate and on our ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Organization and Operations | 138 |
| 對資產徵税。提高有效税率可能會對我們的業務、財務狀況、運營結果和現金流產生不利影響。 近年來,經濟合作與發展組織(OECD) 致力於一個旨在改革國際税收制度的項目,其中包括確保大型跨國企業在其運營的每個司法管轄區 繳納最低水平的税收(支柱二)。2021年12月,經濟合作與發展組織發佈了關於支柱二(全球規則)的示範規則。2022年12月14日,歐盟理事會通過了《(EU)2022/2523號指令》(《歐盟)2022/2523號指令》,該指令針對歐盟內的跨國企業集團和大型國內集團實施全球規則(《第二支柱指令》)。第二支柱指令 要求在2023年12月31日之前在歐盟成員國的國內法中實施。此外,本集團經營業務的某些其他司法管轄區已 已在其國內法中制定全球規則(例如日本),或宣佈 有意在其國內法中實施全球規則。 根據目前的信息,管理層預計本集團最早可能於2025年開始受 第二支柱指令和國內法律的約束。管理層預計《第二支柱指令》和實施的國內法律不會在2024年對本集團產生影響。 本集團目前正在確定對2025年及以後的影響(如果有的話)。 此外,我們可能無法使用,或税務法規的變化可能會影響我們多年來建立的某些未確認税收資產或抵免的使用。例如,我們 在比利時擁有大量重要税收資產,其中一些税收資產可能會因各種交易(包括公司重組或與我們股權結構相關的交易)而被全部或部分沒收,或者它們的使用可能受到相關司法管轄區成文法或解釋的限制。 與美國存託憑證相關的風險因素 我們美國存託憑證和普通股的價格可能會波動, 可能會因我們無法控制的因素而波動。活躍的公開交易市場可能無法持續。 股票市場,尤其是生物製藥公司,經歷了價格和成交量的極端波動,這些波動往往與這些公司的經營業績無關,或與這些公司的經營業績不成比例。2023年,我們在納斯達克上代表普通股的美國存託憑證的收盤價 波動很大,從334.23美元到548.43美元不等。這些證券的交易價格取決於許多因素,包括本“風險因素”部分所述的因素,其中許多因素超出了我們的控制範圍,可能與我們的經營業績無關,這可能會限制或阻止 投資者出售其美國存託憑證或普通股,否則可能對我們的美國存託憑證和普通股的流動性產生負面影響。在公開市場上出售大量美國存託憑證或普通股,或認為這些出售可能會發生,可能會 壓低美國存託憑證和普通股的市場價格,並可能損害我們證券的市場價格或我們通過出售額外股本證券籌集資金的能力。 此外,我們的美國存託憑證或普通股的活躍公開交易市場可能無法持續 。此外,匯率波動還可能影響我們的美國存託憑證和普通股的價格,這可能會導致尋求利用這種差異的投資者進行大量交易,或者影響持有者獲得的收益。 2.10 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 argenx 2023年年度報告美國存托股份|139 |
| If we fail to maintain an effective system of disclosure controls and internal control over financial reporting, our ability to comply with applicable regulations could be impaired, and the trading price of our ADSs may be negatively impacted. We are required to comply with various corporate governance and financial requirements under the Sarbanes-Oxley Act of 2002, the Dodd-Frank Wall Street Reform and Consumer Protection Act, the Nasdaq listing requirements, and other applicable securities rules and regulations. Pursuant to section 404 of the Sarbanes-Oxley Act of 2002, we are required to furnish a report by management on, among other things, the effectiveness of our internal control over financial reporting and an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. Undetected material weaknesses in our internal controls could lead to financial statement restatements and require us to incur the expense of remediation. Moreover, any failure to maintain internal control over financial reporting or any material weaknesses or significant deficiency thereover, could result in a loss of investors’ in the accuracy, completeness and reliability of our financial statements, subject us to sanctions or investigations, or negatively impact the trading price of our ADSs. Holders of our ADSs are not treated as holders of our ordinary shares and may be subject to limitations on the transfer of their ADSs and the withdrawal of the underlying ordinary shares. ADSs are transferable on the books of the depositary. However, holders of ADSs are not treated as holders of our ordinary shares, unless they withdraw the ordinary shares underlying their ADSs in accordance with the deposit agreement and applicable laws and regulations. The depositary may refuse to deliver, transfer or register transfers of ADSs generally when our books or the books of the depositary are closed, or at any time if we or the depositary think it is advisable to do so because of any requirement of law, government or governmental body, or under any provision of the deposit agreement, or for any other reason. Temporary delays in the cancellation of ADSs and withdrawal of the underlying ordinary shares may arise because the depositary has closed its transfer books or we have closed our transfer books, the transfer of ordinary shares is blocked to permit voting at a shareholders’ meeting or we are paying a dividend on our ordinary shares. In addition, ADS holders may not be able to cancel their ADSs and withdraw the underlying ordinary shares when they owe money for fees, taxes and similar charges and when it is necessary to prohibit withdrawals in order to comply with any laws or governmental regulations that apply to ADSs or to the withdrawal of ordinary shares or other deposited securities. Holders of our ADSs do not have the same voting rights as the holders of our ordinary shares and may not receive voting materials in time to be able to exercise their right to vote. Except as described in this Annual Report or any deposit agreements, holders of ADSs are not treated as our shareholders unless they withdraw the ordinary shares underlying their ADSs. The depositary, or its nominee, is the holder of the ordinary shares underlying the ADSs. Holders may vote them in person or by proxy in accordance with applicable laws and regulations and our Articles of Association. We cannot guarantee that holders of ADSs will receive the voting materials in time to ensure that they can instruct the depositary to vote the ordinary shares underlying their ADSs. Our shareholders are only entitled to participate in, and vote at, a general meeting of our shareholders (General Meeting), provided that their shares are recorded in their names at midnight (central European time) at the end of the 28th day preceding the date of such General Meeting. In addition, the depositary’s liability to holders of ADSs for failing to execute voting instructions or for the manner of executing voting instructions is limited by the deposit agreements. As a result, holders of our ADSs may not be able to exercise their right to give voting instructions or to vote in person or by proxy and they ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 American Depository Shares | 140 |
| may not have any recourse against the depositary or us if their ordinary shares are not voted as they have requested or if their shares cannot be voted. If securities or industry analysts cease coverage of us, or publish inaccurate or unfavorable research about our business, the price of our ADSs or ordinary shares and our trading volume could decline. The trading market for the ADSs and ordinary shares depends in part on the research and reports that securities or industry analysts publish about us or our business. If no or too few securities or industry analysts cover us, the trading price of our ADSs and ordinary shares would likely be negatively affected. If one or more of the analysts who cover us downgrade our ADSs or ordinary shares or publish inaccurate or unfavorable research about our business, the price of our ADSs or ordinary shares would likely decline. Risk Factors Related to being a Foreign Private Issuer or a Dutch Company The risks in this subsection that relate to our status as a foreign private issuer will change if we lose our status as a foreign private issuer under U.S. law. We are a Dutch European public company with limited liability (Societas Europaea or SE). The rights of our shareholders may be different from the rights of shareholders in companies governed by the laws of U.S. jurisdictions. We are a Dutch European public company with limited liability. The rights of shareholders and the responsibilities of members of our Board of Directors may be different from the rights and obligations of shareholders in companies governed by the laws of U.S. jurisdictions. As a result of these differences between Dutch corporate law and our Articles of Association, on the one hand, and the U.S. federal and state laws, on the other hand, in certain instances, our shareholders and holders of our ADSs could receive less protection than they would as shareholders or ADS holders of a listed U.S. company. Provisions of our Articles of Association might deter acquisition bids for us that might be considered favorable and prevent or frustrate any attempt to replace or remove the then Board of Directors. Provisions of our Articles of Association may make it more difficult for a third party to acquire control of us or effect a change in our Board of Directors. We have adopted several provisions that may have the effect of making a takeover of our Company more difficult or less attractive. These provisions could discourage potential takeover attempts that other shareholders may consider to be in their best interest and could adversely affect the market price of our securities. Holders of our ordinary shares outside the Netherlands, and holders of ADSs may not be able to exercise pre-emptive rights or preferential subscription rights, respectively. In the event of an increase in our share capital, holders of our ordinary shares are generally entitled under Dutch law to full pre-emptive rights, unless these rights are excluded either by a resolution of the shareholders at a General Meeting, or by a resolution of the Board of Directors (if the Board of Directors has been designated by the shareholders at a General Meeting for this purpose). 2.11 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Being a Foreign Private Issuer or a Dutch Company | 141 |
| However, making pre-emptive rights available to holders of ordinary shares or ADSs representing ordinary shares also requires compliance with applicable securities laws in the jurisdictions where holders of those securities are located, which we may be unable or unwilling to do. In particular, holders of ordinary shares or ADSs located in the U.S. would not be able to participate in a pre-emptive rights offering unless we registered the securities to which the rights relate under the U.S. Securities Act of 1933, as amended (the Securities Act) or an exemption from the registration requirements. In addition, ADS holders would not be able to participate in a pre-emptive rights offering unless we made arrangements with the depositary to extend that offering to holders of ADSs, which we are not required to do. We are not obligated to, and do not comply with, all the best practice provisions of the DCGC, which may affect shareholders’ rights. As a Dutch public company with limited liability, we are subject to the DCGC. We do not comply with all the best practice provisions of the DCGC. As a Dutch company, we are required to disclose in our annual report, filed in the Netherlands, whether we comply with the provisions of the DCGC. If we do not comply with the provisions of the DCGC (for example, because of a conflicting Nasdaq requirement or otherwise), we must list the reasons for any deviation from the DCGC in our annual report filed in the Netherlands. Claims of U.S. civil liabilities may not be enforceable against us or the members of our management and our Board of Directors. Substantially all of our assets are located outside the U.S. The majority of the members of our senior management team and our directors are not U.S. residents and we do not have significant assets in the U.S. As a result, it may not be possible, or may be very difficult, for investors to effect service of process within the U.S. upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the U.S. federal securities laws. There are no treaties between the U.S. with either the Netherlands or Belgium providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S. based on civil liability, whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands or in Belgium unless the underlying claim was re-litigated before a Dutch or Belgian court of competent jurisdiction. This will depend on the applicable Dutch or Belgian national rules. In light of the above, U.S. investors may not be able to enforce any judgments obtained in U.S. courts in civil and commercial matters, including judgments under the U.S. federal securities laws, against us, members of our management or our Board of Directors or certain experts named herein who are residents of the Netherlands, Belgium or countries other than the U.S. In addition, there is doubt as to whether a Dutch or Belgian court would impose civil liability on us or the members of our management or of our Board of Directors in an original action predicated solely upon the U.S. federal securities laws brought in a court of competent jurisdiction against us, our management or directors. As a foreign private issuer, we are exempt from certain rules under U.S. securities laws and are permitted to file less information with the SEC than a U.S. company. As a “foreign private issuer” defined in the SEC’s rules and regulations, we are exempt from certain provisions of the Exchange Act that are applicable to U.S. domestic public companies. We are subject to Dutch laws and regulations with regard to such matters. While we furnish quarterly unaudited financial information to the SEC on Form 6-K, the information we furnish to the SEC is less extensive and less timely compared to that required to be filed with the SEC by U.S. domestic issuers. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Being a Foreign Private Issuer or a Dutch Company | 142 |
| As a foreign private issuer, we are permitted to adopt certain home country governance practices rather than the corporate governance requirements of Nasdaq. These practices may afford less protection to shareholders than they would enjoy if we complied fully with corporate governance listing standards. As a foreign private issuer listed on Nasdaq, we are subject to corporate governance listing standards. However, we are permitted to rely on home country governance requirements and certain exemptions thereunder. Certain of our corporate governance practices may differ significantly from other corporate governance listing standards. We may lose our foreign private issuer status which would then require us to comply with the Exchange Act’s domestic reporting regime and cause us to incur significant legal, accounting and other expenses. In order to maintain our current status as a foreign private issuer, either (a) a majority of our ordinary shares must be either directly or indirectly owned of record by non-residents of the U.S. or (b)(i) a majority of our executive officers or directors may not be U.S. citizens or residents, (ii) more than 50% of our assets cannot be located in the U.S. and (iii) our business must be administered principally outside the U.S. As of February 1, 2024, we believe at least 50% of our outstanding ordinary shares were held by U.S. residents (assuming that all our ordinary shares represented by ADSs were held by residents of the U.S.). The regulatory and compliance costs to us as a U.S. domestic issuer may be significantly higher than those we incur as a foreign private issuer. We also expect that if we were required to comply with the rules and regulations applicable to U.S. domestic issuers, it would make it more difficult and expensive for us to obtain director and officer liability insurance, and we may be required to accept reduced coverage or incur substantially higher costs to obtain coverage. These rules and regulations could also make it more difficult for us to attract and retain qualified members of our Board of Directors. If we were to be classified as a passive foreign investment company for U.S. federal income tax purposes, this could result in adverse U.S. tax consequences to certain U.S. holders. If our Company is classified as a passive foreign investement company (PFIC) for any taxable year, U.S. investors may be subject to adverse U.S. federal income tax consequences described below under “Taxation – U.S. Federal Income Tax Considerations – Passive Foreign Investment Company Rules”. Our Company will be classified as a PFIC for U.S. federal income tax purposes for any taxable year in which, taking into account a pro rata portion of the income and assets of 25% or more owned subsidiaries, either (i) at least 75% of its gross income consists of “passive income” or (ii) at least 50% of the average quarterly value of its assets is attributable to assets that produce, or are held for the production of, passive income. Based on our historic and anticipated operations, the composition of our income and the projected composition and estimated fair market values of our assets, we do not believe that we were a PFIC for our most recent taxable year and do not expect to be classified as a PFIC for the current taxable year or for the foreseeable future. However, our status as a PFIC is a factual determination made on an annual basis, and we cannot provide any assurances regarding our PFIC status for the current or future taxable years. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Being a Foreign Private Issuer or a Dutch Company | 143 |
| 公司 管治 3.1荷蘭公司管治守則145 3.2管理架構146 3.3非執行董事報告172 3.4薪酬報告及薪酬 聲明176 3.5企業管治—納斯達克上市規則217 3.6股權所有權218 3.7內幕交易218 3.8網絡安全218 3.9風險偏好及控制220 3 arginx 2023年度報告公司管治|144 |
| 3公司治理 荷蘭公司治理守則 作為一家根據荷蘭法律註冊成立的歐洲上市公司(Societas Europaea),我們受荷蘭公司治理守則(DCGC)的約束。可在www.mccg.nl上找到DCGC的副本。DCGC基於這樣一個概念,即公司是公司各利益相關者之間的長期聯盟。利益相關者是指直接或間接影響或受其影響實現我們的目標的團體和個人:員工、股東和其他貸款人、供應商、客户和其他 利益相關者。我們的董事會有責任權衡這些利益,通常是 ,以確保我們和我們的子公司的連續性,因為我們尋求創造長期 價值。如果利益相關者要在公司內部和與公司合作,他們需要 確信他們的利益得到了適當的考慮。良好的企業家精神和有效的監督是利益相關者對管理和監督的信心的必要條件。這包括董事會行為的完整性和透明度,以及對董事會監督的責任。 DCGC基於“要麼遵守,要麼解釋”的原則。因此,公司必須在其年度報告中説明它們在多大程度上遵守了DCGC的原則和最佳實踐條款,如果它們不遵守這些原則和最佳實踐條款,則説明它們為什麼以及在多大程度上偏離這些原則。 我們認識到良好公司治理的重要性,我們完全贊同DCGC的基本原則,這反映在符合DCGC(董事會章程)所述最佳實踐條款的政策中。董事會章程可在我們的網站(www.argenx.com/Investors)上查閲。但是,出於本節中解釋的原因,我們偏離了以下領域的最佳實踐規定。這些 偏差均與我們的薪酬實踐有關,符合我們在2021年召開的年度股東大會批准的薪酬政策。 ·根據DCGC vi項下的最佳實踐條款3.1.2,股票應在授予股票後至少持有 五年。雖然我們確實有最低持股要求 要求我們的董事和執行管理層在其任職期間和之後的一段時間內持有公司的最低所有權水平,但我們 在授予股票後5年內對出售股票沒有一般性限制。 我們定期對我們的股權激勵做法進行基準測試,並注意到授予後5年的全面出售 限制明顯比我們絕大多數競爭對手對人才實施的出售限制 嚴格得多。我們認為,我們對RSU的總體歸屬期限為4年,對股票期權的歸屬期限為3年,再加上歸屬期限後的最低持有量要求 ,有效確保長期利益一致 我們預計在可預見的未來不會對所有股權實施一般的5年持股要求。 3.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告荷蘭公司治理代碼|145 |
| · Pursuant to best practice provision 3.2.3. of the DCGC, the severance payment in the event of dismissal should not exceed one year’s base compensation. Our remuneration policy provides that a severance payment equal to 18 months base compensation to our chief executive officer (CEO). The severance component of the remuneration package is, like all other components, benchmarked against and aligned with the severance components as identified within the reference group. On this topic, considering the importance of competitive remuneration for our ability to attract and retain highly qualified persons, alignment with the reference group is prioritized over compliance with this best practice provision 3.2.3. We currently do not envision to change our practice in this respect. · Pursuant to best practice provision 3.3.2. of the DCGC, non-executive directors should not be granted any shares or rights to shares as remuneration. We note that the ‘best practices’ and usages regarding granting equity incentives to non-executive directors vary significantly between the key jurisdictions in which we operate. For example, we conduct a significant part of our operations in Belgium and the Belgian Corporate Governance Code requires that non-executive directors receive part of their remuneration in the form of shares, but not stock options. Our benchmarking confirms that offering equity incentives to non-executive directors in the form of options and/or shares is on the other hand widely accepted market practice in the U.S., with the majority of our U.S. reference group companies granting stock options to directors (benchmark of September 2023). We believe it is in the interest of our stakeholders that we are equipped to recruit the talent on our Board of Directors proportionate to our international ambitions. For this reason, we aligned our remuneration practices with those prevalent in the key markets in which we need to compete for talent. Considering specifically our significant activities in the U.S. and the specialized knowledge and experience needed on our Board of Directors to maximize our chances of success in this region, we need to align our remuneration practices for non-executive directors with the U.S. companies in our reference group, meaning we offer share options and/or restricted share units to our non-executive directors. This is a conscious and well-considered deviation from the DCGC that we believe is required to serve our long-term global goals and ambitions. On this topic, considering the importance of competitive remuneration for our ability to attract and retain highly qualified persons, alignment with the reference group is prioritized over compliance with this best practice provision 3.3.2. We currently envisage several changes to our remuneration policy, but we expect to continue to offer some form of equity remuneration to our directors in the foreseeable future, unless the practice in our reference group changes. If our benchmark exercise shows that offering only cash (no equity incentives), we will consider adhering in full to this best practice provision. Management Structure General As at December 31, 2023, we have a one-tier board structure consisting of one executive director and eight non-executive directors, and a senior management team (consisting of our CEO and senior personnel reporting directly to the CEO) responsible for the day-to-day operations. We have opted for this structure to allow for a division of responsibilities between our Board of Directors and our senior management team, keeping our Board of Directors at a manageable size whilst being able to involve some or all members of our senior management team in discussions with the Board of Directors if and when necessary. In practice, all members of our senior management team are regularly involved in the discussions of our Board of Directors and its committees, in order to provide information and context to the various issues the Board of Directors needs to decide on. In addition to being 3.2 3.2.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Management Structure | 146 |
| present at meetings from time to time, our senior management and other senior leaders in the organization keep regular contact (face to face or via electronic means) with members of the Board of Directors and its committees. Set out below is a summary of certain provisions of Dutch corporate law as of the date of this Annual Report, as well as a summary of relevant information concerning our Board of Directors and certain provisions of our articles of association (Articles of Association) and the Board By-Laws. This summary does not purport to give a complete overview and should be read in conjunction with and is qualified in its entirety by reference to the relevant provisions of Dutch law as in force on the date of this Annual Report, the Articles of Association and Board By-Laws. The Articles of Association are available in the governing Dutch language and an unofficial English translation thereof, and the Board By-laws are available in English, on our website. Statement of the Board of Directors Responsibilities for the Financial Statements and Management Report In accordance with Article 5:25c(2)(c) of the Dutch Financial Supervision Act (Wet toezicht financiële verslaggeving) (DFSA), the Board of Directors hereby certifies that, to the best of our knowledge, our consolidated financial statements as of December 31, 2023, prepared in accordance with International Financial Reporting Standards (IFRS) as adopted by the EU, and with the legal requirements applicable in the Netherlands, give a true and fair view of the assets, liabilities, financial position and profit or loss of the company and the undertakings included in the consolidation taken as a whole, and that the management report includes a fair review of the development and performance of the business and the position of argenx and the undertakings included in the consolidation taken as a whole, together with a description of the principal risks and uncertainties that they face. Responsibility for this Annual Report The Board of Directors declares that the information contained in this Annual Report, including our consolidated financial statements as of December 31, 2023 and the management report, is, to the best of its knowledge, in accordance with the facts and contains no omission likely to affect its import. The Board of Directors is responsible for the information given in this Annual Report. In Control Statement Our Board of Directors is responsible for the oversight of our risk management activities and has specifically designated the audit and compliance committee to assist our Board of Directors in this task and prepare recommendations in this respect to the Board of Directors. While our Board of Directors oversees our risk management, our senior management is responsible for day-to-day risk management processes. Our Board of Directors expects our senior management to consider risk and risk management in each business decision, to proactively develop and monitor risk management strategies and processes for day-to-day activities and to effectively implement risk management strategies adopted by the Board of Directors. We believe this division of responsibilities is the most effective approach for addressing the risks we face. 3.2.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Statement of the Board of Directors | 147 |
| Board of Directors Responsibilities Pursuant to the Dutch Civil Code (DCC), our Board of Directors is collectively responsible for our general affairs. Our Board of Directors, our executive director as well as our non-executive directors, define our strategy (as further set out in section “Strategy and objectives”). Our strategy is regularly discussed and monitored at our Board of Directors meetings. The Board recognizes the critical importance of robust ESG practices for sustainable business operations and long-term value creation. Accordingly, the Board is committed to ensuring comprehensive ESG reporting and oversight. This includes the responsibility to develop and implement effective ESG strategies, oversee the integration of ESG considerations into corporate decision-making and ensure transparent and accurate disclosure of ESG performance to stakeholders. The Board regularly reviews and assesses the Company’s ESG-related risks and opportunities, ensuring alignment with legal requirements, industry standards and stakeholder expectations. In fulfilling this role, the Board engages with relevant internal and external stakeholders to inform its strategies and decisions, fostering a culture of ESG excellence throughout the organization. Pursuant to our Articles of Association, our Board of Directors will divide its duties among its members, with our day-to-day management entrusted to the executive director(s). The non-executive directors are tasked with supervising our management and advising the executive director(s). In addition, both the executive director(s) and the non-executive directors must perform the duties assigned to them pursuant to the Articles of Association. The division of tasks within our Board of Directors is determined (and amended, if necessary) by our Board of Directors. Our executive director(s) may not (i) serve as chairperson of our Board of Directors; (ii) determine the remuneration of an executive director or (iii) nominate directors for appointment. Each director has a duty to properly perform the duties assigned to him or her and to act in our corporate interest. Under Dutch law, the corporate interest extends to the interests of all corporate stakeholders, such as shareholders, creditors, employees and other stakeholders. Composition, Appointment and Dismissal The Articles of Association provide that our Board of Directors will consist of our executive director(s) and non-executive directors. The number of executive directors must at all times be less than the number of non-executive directors. The number of directors, as well as the number of executive directors and non-executive directors, is determined by our Board of Directors, provided that the Board of Directors must consist of at least three members. Our directors are appointed by the shareholders at a General Meeting for a period of four years as either executive directors or as non-executive directors. In accordance with best practice provision 2.2.1 of the DCGC, executive directors may be reappointed for periods not more than four years at a time. In accordance with best practice provision 2.2.2 of the DCGC, non-executive directors may be reappointed once for a period of four years , after which the non-executive director may be reappointed again for a period of two years, which reappointment may be extended by at most two years. In the event of a reappointment after an eight-year period, reasons will be given in the report of the Board of Directors. The Board of Directors is required to make one or more proposals for each seat on our Board of Directors to be filled. A resolution to nominate a director by our Board of Directors (with support from the remuneration and nomination committee) may be adopted by a simple majority of the votes cast. A nomination for appointment of an executive director must state the candidate’s age and the positions he or she holds, or has held, insofar as these are 3.2.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 148 |
| 與董事高管履行職責相關。提名必須載明提名有關人士的理由。任命非執行董事的提名必須説明候選人的年齡、他或她的專業、他或她持有的股份數量以及他或她所擔任或曾經擔任的僱傭職位, 這些與履行非執行董事的職責有關。此外,應註明他或她已經是監事會成員或非執行董事的法人實體的名稱;如果這些法人實體包括屬於同一集團的法人實體,則提及該集團就足夠了。提名必須 説明提名相關人員的理由。 我們董事會指定董事高管一人為首席執行官。此外,董事會還可以授予執行董事其他頭銜。我們的董事會還任命 一名非執行董事董事擔任董事會主席,並任命一名非執行董事董事 擔任董事會副主席。董事會執行成員與Argenx之間的法律關係不被視為僱傭協議 。允許董事高管與集團公司 (Argenx SE除外)簽訂僱傭協議。在沒有僱傭協議的情況下,董事會成員通常不享有與荷蘭勞動法規定的員工同等的保護。 有關當前任期屆滿日期和該人在該職位上任職的時間的討論,請參閲“非執行董事”部分和 “高級管理人員”部分。 除“關聯方交易”部分所述的安排外, “與我們的高級管理人員的協議”,除適用法律所規定外,吾等與任何執行董事之間並無任何安排或諒解 就終止聘用後的福利作出任何規定。此外,我們 與我們的非執行董事之間的合同不提供終止時的任何福利。 作為一家外國私人發行人,根據納斯達克的上市要求和規則,我們的董事會並不要求 必須有過半數的獨立董事,但我們的 審計與合規委員會必須完全由獨立董事組成。 但是,我們的董事會決定,考慮到任何適用的 委員會獨立性標準,我們的所有非執行董事,包括我們的審計與合規委員會的 成員,根據《交易所法案》規則 10A-3以及納斯達克和星展中心的適用規則,為“獨立董事”。在做出這樣的決定時,我們的董事會考慮了每一位非執行董事與我們的關係,以及我們的董事會 認為與確定董事獨立性相關的所有其他事實和情況,包括董事及其關聯實體(如果有)實益擁有的普通股數量。 董事董事會要求非執行董事的組成能夠使成員 能夠相互獨立且關鍵地運營,執行董事 以及所涉及的任何特定利益。截至本年度報告日期,所有非執行董事 均符合DCGC所載的獨立性標準。因此,非執行董事認為,我們的非執行董事的組成符合DCGC最佳實踐條款2.1.7至2.1.9的獨立性要求。因此,我們的董事會還決定,根據DCGC. ar Gr g oup enx Factors Risk Go Corporate vernance資本的適用規則,我們委員會的所有成員都是獨立的 股票財務 審查報表 財務非財務 信息 Argenx董事會2023年年度報告|149 |
| As of the date of this Annual Report (or in any period before), none of the members of our Board of Directors and senior management has or has had a family relationship with any other member of our Board of Directors or senior management. Directors may be suspended or removed by the shareholders at a General Meeting at any time, with or without cause, by means of a resolution passed by a simple majority of the votes cast. Pursuant to the DCC, executive directors may also be suspended by the board of directors. A suspension of an executive director by the board of directors may be discontinued by the shareholders at any time at a General Meeting. Diversity We value diversity among our colleagues as an integral component in building a sustainable growth platform and believe that a diverse workforce enhances our overall performance and success. We take pride in creating and sustaining a culture and environment where each of us can excel. We bring together people with diverse backgrounds experiences and functional expertise. By doing so, we broaden the scope of ideas and creativity essential to developing and delivering innovative therapies to patients. Acknowledging and benefiting from different perspectives promotes diversity of thought and empowers innovation. It also contributes to our commitment to improve the quality of lives of patients, wherefore we need teams with a healthy mix of contrasting perspectives and backgrounds that reflect the diverse communities we serve. We recognize that our people are our greatest strength. Fostering an inclusive work environment where everyone feels safe and encouraged to contribute leads to better work outcomes and supports high levels of employee commitment and retention. We aspire to be a consciously global company. Our success is built on, and dependent on true collaboration in cross-functional and often cross-regional teams in which open communication is encouraged and safeguarded. Everyone has a voice and is encouraged to contribute to the benefit of our common goals, irrespective of race, ethnicity, age, gender or cultural background. Good ideas as well as real concerns are taken seriously, regardless of who brings them forward. We aim to foster an inclusive work environment in support of our strategic plan and priorities. We continue to raise the bar in this regard, and to commit to measures and goals designed to support our maturing company culture. We have set ourselves the goal of gender balance across all levels at argenx, including our Board of Directors. In 2022, we adopted our current diversity, equity and inclusion policy, which sets out the basis for our inclusion, equity and diversity management throughout our organization in a way that we believe best supports our business objectives and our people. We monitor and annually report on relevant diversity, equity and inclusion metrics, initiatives and developments in this Annual Report and in our ESG reports, of which an updated version will be published on or around the date of this Annual Report. Our policy is that we aim to balance our Board of Directors and senior management team in terms of gender, age, background, race, ethnicity, sexual orientation, experience and nationality as much as reasonably possible while still having our Board of Directors and senior management team composed of the best possible candidates overall. It has been and will remain our priority to have the best available specialists on our Board of Directors and in our senior management team, who make a balanced panel of directors and managers able to advise and guide argenx to further growth and success for all its stakeholders. This means we require a number of specialties and character traits to be present. We will seek to further improve diversity on our Board of Directors if and when proposing new appointments to our Board of Directors, whilst recognizing that, considering the specialist nature of our business, aspects other than diversity are relevant as well for the ultimate decision to select a board member. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 150 |
| Our plan of action to achieve our goal of gender balance includes a number of recruitment and development-related initiatives to promote balanced and diversified candidate pools as well as diversity amongst persons receiving promotion and development opportunities. We value our fair, inclusive recruitment process, which is standardized across the organization and focuses on pre-identified ‘what counts’ factors. The process involves a diverse group of colleagues from across the organization, who are provided with training to recognize existing biases. Recruitment decisions are based on a group evaluation of available candidates, to encourage different perspectives. Our onboarding program is designed to promote inclusion by building a strong social fabric across teams, functions and geographic locations. Once hired, employees are encouraged to participate in a personal development program aimed at building on their individual strengths to benefit the broader team and taking into account their individual career aspirations. We offer opportunities for promotion, training and career development solely based on job-related, appropriate criteria such as skills, competencies, experience, aptitude and enthusiasm and giving account to each individual’s experience, ambitions and capabilities. We will continue to implement our diversity, equity and inclusion policy by seeking new ways to improve and support diversity, equity and inclusion at the Company. We from time to time report on specific initiatives taken with respect to our diversity, equity and inclusion policy in our annual ESG report, of which an updated version will be published on or around the same date as this Annual Report. In accordance with Dutch legislation, we report annually to the Social Economic Council (Sociaal-Economische Raad) whether or not we have complied with our diversity goals, and if we have not, the reasons for this. As at December 31, 2023, our Board of Directors consisted of nine directors, including one executive director and eight non-executive directors. Of the directors who chose to disclose their gender, the Board of Directors contained five male directors and three female directors (non-executive directors), translating into a 55.55% male/331/3% female balance for our full Board of Directors (compared to five males and three females (non-executive directors) (55.55%/331/3%) as of December 31, 2022) and a 62.5% male/37.5% female balance for our non-executive directors (compared to 62.5% male/37.5% female as of December 31, 2022). As at December 31, 2023 and December 31, 2022, our Company leadership team consisted of 31 persons, comprised of a mix of 19 males and 12 females, (61%/39% respectively). Our leadership consists of all full time employees reporting directly to our CEO, as well as all (other) leaders of our largest functions and projects. Each of these positions is characterized by a high impact across the organization, leading a global and cross functional team and having a global reach. We estimate that as of December 31, 2023, 58% of our workforce were female and 42% were male (compared to 63% female and 37% male as of December 31, 2022). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 151 |
| 董事會多樣性矩陣(截至本年度報告日期) 主要執行辦公室所在的國家/地區荷蘭 在美國的外國私人發行人。是 荷蘭法律禁止披露性別身份 董事總數9性別: 女性: 3男性: 5非二進制: 0未披露 性別身份: 1個人口統計背景類別 每個人口統計類別中的董事數量 類別{Br}在母國司法管轄區1 LGTBQ+0任職人數不足的個人沒有披露人口統計背景8會議和決策 我們的董事會章程,其中規定了召開董事會會議的程序,以供董事會決策和董事會的運作程序。根據我們的公司章程,我們的董事會至少每三個月召開一次 會議,討論公司的內部事務狀況和預期的 發展。 根據我們的董事會章程,我們的董事會成員必須在可能的情況下努力確保決議獲得一致通過。如果無法達成一致,且荷蘭法律、公司章程或董事會章程未規定獲得較大多數,董事會的所有決議必須在至少有在任董事會成員出席或派代表出席的會議上以簡單多數 通過。公司章程規定,在票數相等的情況下,主席沒有決定性的投票權,因此,在票數相等的情況下,提案將被否決。 根據董事會章程,某些具體事項需要非執行董事的多數批准。這些事項載於本局附例附表1。我們的董事會 章程可在我們的網站上找到。非執行董事也可以決定,其他某些事項需要獲得非執行董事一定多數的批准。應明確規定,並書面通知董事(S)執行董事。 董事會決議也可以在非書面會議上通過, 只要所有在任董事(公司章程中提及的董事不存在利益衝突)均已書面同意這種決策方式。 一家董事可以 書面向另一家董事出具特定董事會會議的委託書。 董事與 公司及其關聯企業的利益存在直接或間接的個人利益衝突。每個董事應向all ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 argenx董事會2023年年度報告|152 |
| other directors of a conflict of interest without delay. A director shall not participate in the deliberations and decision-making process in relation to an item if he has a conflict of interest with respect thereto. In such case, the other directors shall resolve the item. In case because of this no resolution can be adopted by the executive directors, the non-executive directors will resolve on the matter. In case because of this no resolution can be adopted by the non-executive directors, the Board of Directors will resolve on the matter as if there were no conflict of interest. The executive director(s) are required to be asked their vision on their own remuneration in accordance with best practice provision 3.2.2 but may not participate in the adoption of resolutions (including any deliberations in respect of such resolutions) relating to their remuneration. Committees In accordance with the DCGC, our non-executive directors can set up specialized committees to analyze specific issues and advise the non-executive directors on those issues and prepare resolutions with respect thereto. The committees are advisory bodies only, and the decision-making remains within the collegial responsibility of the Board of Directors. The non-executive directors determine the terms of reference of each committee with respect to the organization, procedures, policies and activities of the committee. Our non-executive directors have established and appointed (i) an audit and compliance committee; and (ii) a remuneration and nomination committee. The composition and function of these committees complies with all applicable requirements of Euronext Brussels, the DCGC, the Exchange Act, the exchange on which the ordinary shares and the ADSs are listed and U.S. SEC rules and regulations. Only non-executive directors qualify for membership of these committees. The audit and compliance committee and the remuneration and nomination committee may not be chaired by the chairperson of the Board of Directors or by a former executive director of the Company. In addition to the aforementioned legally required subcommittees, our Board of Directors may also opt to incorporate informal committees consisting of non-executive directors and other internal and external persons in argenx, in order to facilitate discussions and act as a sounding board on specific projects, as well as on a more permanent basis. Our Board of Directors has incorporated a research and development committee and a commercialization committee. Audit and Compliance Committee Our audit and compliance committee consists of four members: Steve Krognes (chairperson), effective February 27, 2023, Peter K. M. Verhaeghe, Anthony A. Rosenberg and James M. Daly. Mr. Lanthaler was a committee member and chairperson until February 27, 2023. Our Board of Directors previously established that Mr. Verhaeghe, Mr. Rosenberg, Mr. Daly, Mr. Krognes satisfy the independence requirements set forth in Rule 10A-3 of the Exchange Act and that both Mr. Lanthaler (up until his resignation effective February 27, 2023) and Mr. Krognes qualify as “audit committee financial experts” as defined by SEC rules and Article 39 paragraph 1 of Directive 2014/56/EU of the European Parliament and of the Council of April 16, 2014 amending Directive 2006/43/EC on statutory audits of annual accounts and consolidated accounts and has the requisite financial sophistication under the applicable Nasdaq rules and regulations. Further, our Board of Directors established that the ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 153 |
| 審計和合規委員會的組成符合荷蘭關於設立審計委員會的法令的要求。 我們的審計和合規委員會協助我們的董事會監督我們的會計和財務報告流程的準確性和完整性,以及對我們的綜合財務報表和非財務報表(包括ESG 報告)的審計和審查,內部控制系統的實施和有效性,以及我們 遵守法律和法規要求的情況,獨立審計師的資格和獨立性以及獨立審計師的表現。我們的審核和合規委員會還負責監控我們的全球道德和合規計劃的狀態和合規情況,並至少每季度與我們的道德和合規部門負責人會面,討論 計劃的狀態和總體有效性,以及發生的任何問題或事件和所需的補救措施(如果 適用)。此外,該委員會還監督與氣候有關的風險,監督公司網絡安全計劃的 狀況,並定期(至少每季度)與我們的高級管理團隊討論 該計劃的狀況。 我們的審計與合規委員會受一份章程管轄,該章程遵守《納斯達克全球市場上市規則》(《納斯達克上市規則》)和國家發改委的章程,並可在我們的網站上公開 查閲。它負責制定方法和程序,以監督我們的財務報告、風險管理、道德和合規以及組織,並在必要時要求改進,以便就此向我們的董事會提出適當的建議。 我們的審計和合規委員會根據其正常運作所需定期開會, 但每年至少四次,至少每年一次,分別與我們的獨立審計師 會面。有關 會議次數和出席率的概述,請參閲“報告審核和合規委員會”一節。 我們的審核和合規委員會定期向董事會報告其職能的行使情況。它向我們的董事會通報其認為需要採取行動或改進的所有領域,並就需要採取的必要步驟或決議提出建議。審核審核和報告 審核涵蓋我們和我們的子公司作為一個整體。審核和合規委員會的成員有權從我們的董事會和員工那裏獲得履行其職能所需的所有信息。審計與合規委員會的每名成員應與審計與合規委員會主席協商行使這一權利。 薪酬與提名委員會 我們設立了薪酬與提名委員會,作為 DCGC規定的 薪酬委員會和選拔任用委員會。我們的薪酬和提名委員會目前由三名成員組成:J. Donald de Bethizy(主席)、Peter K.M.Verhaeghe和Ana Cespedes。 我們的薪酬和提名委員會負責除其他事項外: ·根據所有相關的 情況和基準定期審查薪酬政策和做法,並向非執行董事建議個別執行董事的薪酬; ·就非執行董事的薪酬向董事會提供建議; ·準備要包括在我們年度report; ar Gr g oup enx Factors Risk Go Corporate vernance資本中的薪酬報告 股票財務 審查報表 財務非財務 信息 Argenx董事會2023年年度報告|154 |
| · drawing up selection criteria and appointment procedures for directors and making proposals for appointment and re-appointment of the directors; · periodically assessing the size and composition of our Board of Directors and making a proposal for a composition profile of the non-executive directors; · periodically assessing the diversity (including gender diversity) on our Board of Directors and leadership teams, and taking into account any gaps between our then current diversity metrics and the goals specified in our diversity, equity and inclusion policy when making recommendations to the Board of Directors; · periodically assessing the functioning of individual directors and reporting on this to the non-executive directors; and · supervising the policy of the executive directors on the selection criteria and appointment procedures for senior management. In addition, our remuneration and nomination committee takes into account ESG when performing its duties, making sure that (i) ESG performance metrics are incorporated in the remuneration, (ii) ESG qualifications, experience, and expertise are taken into account in the director and executive nomination process, (iii) a culture of awareness and accountability for non-financial performance metrics is promoted, (iv) a diverse, equitable, and inclusive work environment is fostered, (v) our ESG reporting is in line with applicable regulatory requirements and industry best practices in these specific areas, and (vi) we maintain constructive dialogue with key stakeholders on ESG matters. The remuneration and nomination committee consists of at least three members. The remuneration and nomination committee meets as often as is required for its proper functioning, but at least once per year to evaluate its functioning. Please refer to section “Report Remuneration and Nomination Committee” for an overview of the number of meetings and attendance rates. Informal subcommittees Research and development committee The research and development committee consists of members of our Board of Directors and other persons, which composition may vary from time to time. Currently, the research and development committee consists of two members who are also members of our Board of Directors: J. Donald deBethizy and Pamela Klein. Non-board member advisors of the research and development committee include David Lacey, Hans de Haard and Wim Parys. Ad-hoc participants to the committee meetings include a variety of employees and/or external advisors, depending on the needs of the committee and the topics under discussion. The research and development committee is responsible for, among other things: · monitoring and overseeing our research and development goals, strategies and measures; · serving as a sounding board to our research and development management, general management and Board of Directors; · performing strategic reviews of our key research and development programs; · reporting to our Board of Directors on the outcome of the strategic reviews; · reviewing our scientific publication and communications plan; · evaluating and challenging the effectiveness and competitiveness of our research and development endeavors; · reviewing and discussing emerging scientific trends and activities critical to the success of our research and development; · reviewing our clinical and preclinical product pipeline; and ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 155 |
| · engaging in attracting, retaining and developing our senior research and development personnel. The research and development committee also pays specific attention to ESG duties when performing its duties. Amongst others, it help ensure that we meet our commitment to ensuring animal testing is carried out only when necessary and when no alternative methods are reasonably available and that we have policies and procedures in place to support high standards of animal welfare, minimizing pain and distress to research animals. Furthermore, it ensures a transparent reporting on animal testing practices and in R&D practices and helps ensure the prioritization of safety, dignity, and rights of clinical trial participants that informed consent is obtained in a transparent and ethically sound manner. All members of the research and development committee shall have adequate industrial, academic and/or practical experience with the research and development of biopharmaceuticals. One purpose of our research and development committee is to engage in discussion with our research and development personnel, and the committee’s responsibilities to carry out this purpose include, among others: monitoring the research and development activities, performing strategic reviews of the key research and development programs and reviewing the scientific publication plan, all with the intent to support our innovation mission. Our research and development committee meets as often as is required for its proper functioning, but typically meets at least once prior to each meeting of our Board of Directors and reports regularly to our Board of Directors on the outcome of its deliberations, including any recommendations to the Board of Directors or the senior management team. The chairperson of our research and development committee reports to our Board of Directors on the research and development committee’s discussions and strategic advice after each meeting on all matters within its duties and responsibilities. Please refer to section “Report Research and Development Committee” for an overview of the number of meetings and attendance rates. Commercialization committee Our commercialization committee consists of members of our Board of Directors and other persons, which composition may vary from time to time. As of the date of this Annual Report, the commercialization committee consists of three permanent members: James M. Daly (chairperson), Anthony A. Rosenberg and Camilla Sylvest. Keith Woods serves as a non-board member advisor of the committee. The commercialization committee is responsible for, among other things: · reviewing and guiding the global sales and marketing strategy to ensure optimal product uptake and sustained growth and promoting innovation within commercialization efforts; · overseeing the global product launch strategy and supervising all stages of product lifecycle; · reviewing our partnerships and collaborations; · reviewing and guiding the Company’s global medical affairs strategy; · identifying and advising on potential risks associated with commercialization strategies and ensuring commercial strategies adhere to regulatory obligations; · reviewing the ESG reporting on the topics which are relevant to the activities of the committee and providing comments thereto; and · reporting to our Board of Directors on the outcome of the strategic reviews. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Board of Directors | 156 |
| The non-executive directors shall appoint and dismiss the members of the commercialization committee. All members of the commercialization committee shall have adequate industrial, academic and/or practical experience with the commercialization of (bio)pharmaceuticals. Our commercialization committee meets as often as is required for its proper functioning and in practice meets at least once per quarter. The commercialization committee reports regularly to our Board of Directors on the outcome of its strategic reviews and any recommendations to the Board of Directors or senior management team. Please refer to section “Report Commercialization Committee” for an overview of the number of meetings and attendance rates. Non-Executive Directors Our Board of Directors as at December 31, 2023 comprised the following eight non-executive directors: Peter K. M. Verhaeghe Peter Verhaeghe has served as a member and chairperson of the board of arGEN-X B.V. since October 2008 and as non-executive director on our Board of Directors since July 2014. Mr. Verhaeghe is the managing partner of VVGB Advocaten-Avocats, a corporate finance law and tax law firm, a position he has held since July 1999. He is currently lead counsel to a number of Belgian, Dutch, French, U.S. and Swiss life sciences companies. Mr. Verhaeghe has served on the boards of directors of Participatiemaatschappij Vlaanderen NV since May 2018 and miDiagnostics NV since April 2020. He has also served as chairman of the board of Haretis SA (Luxembourg) since March 2011 and as chairman of the LP & advisory committee of Bioqube Factory Fund I NV since September 2020. Mr. Verhaeghe previously served as a member of the board of directors of CzechPak Manufacturing s.r.o., Innogenetics NV (now Fujirebio Europe N.V.), Tibotec-Virco NV, and Biocartis SA. He was also the president of the board of directors of Merisant France SAS, a member of the management board of Merisant Company 2 sàrl., and chairman of the board of directors of PharmaNeuroBoost NV. Mr. Verhaeghe holds a degree in law from the University of Leuven and an LL.M. degree from Harvard Law School. 3.2.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 157 |
| Dr. Werner Lanthaler (until February 27, 2023) Dr. Werner Lanthaler served as a member of our Board of Directors from July 2014 until February 27, 2023. Dr. Lanthaler served as the CEO of Evotec SE until January 2024, a global drug discovery and development organization, a position he held since March 2009. He also serves on the supervisory board of AC Immune SA (Switzerland). Dr. Lanthaler holds a degree in psychology, a Ph.D. in business administration from Vienna University of Economics and Business, and a Master’s degree in public administration from Harvard University. Mr. Steve Krognes (effective February 27, 2023) Mr. Krognes has served as a member of our Board of Directors and as a chairperson of our audit and compliance committee since February 27, 2023. Mr. Krognes also serves on the boards of directors of Guardant Health, Inc., Denali Therapeutics, Inc., and Gritstone bio, Inc. In September 2023, he also was appointed to the board of directors of ClayvstBio. He previously served on the boards of directors of RLS Global AB and Corvus Pharmaceuticals, Inc. Mr. Krognes was the founding chief financial officer of Denali Therapeutics, Inc. (Denali Therapeutics), from 2015 until retiring from that position in April 2022. In that role, he built and led the finance team as well as supervising the IT and facilities functions. He then joined the board of directors of Denali Therapeutics. Mr. Krognes led successful financings for Denali Therapeutics, including its initial public offering in 2017, and contributed significantly to the company’s strategy, growth and strong financial position. His extensive leadership experience in the biotech and pharmaceutical industries includes 12 years in total at Roche and Genentech, Inc., during which Mr. Krognes served as chief financial officer of Genentech, Inc. for six years and global head of Roche’s mergers & acquisition team for six years. He also chaired the Genentech Access to Care Foundation and represented Genentech on the board and executive committee of the California Life Science Association. Before that, Mr. Krognes worked as an investment banker at Goldman Sachs, as a management consultant at McKinsey & Company, and as a venture capitalist in Scandinavia. Mr. Krognes holds a Master’s of Business Administration (MBA) from Harvard Business School and a Bachelor’s of Science in economics from the Wharton School of the University of Pennsylvania. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 158 |
| Dr. J. Donald deBethizy Dr. deBethizy has 30 years of experience in research and development, as well as financial, business and operating management, and board work in the biotechnology and consumer products industries. He is the president of White City Consulting ApS, a consulting company that specializes in advising technology-focused companies. Dr. deBethizy currently serves on the boards of directors of Lophora ApS and Proterris, Inc. and as a board advisor for Cereno Scientific AB. Previously, Dr. deBethizy served as president and CEO of Santaris Pharma A/S until October 2014, when the company was sold to Roche. From March 1997 to June 2012, Dr. deBethizy was co-founder and CEO of Targacept Inc. (Targacept), a U.S. biotechnology company listed on Nasdaq. From June 2012 to May 2013, he was special advisor to the chairman of Targacept’s board of directors. From May 2013 to November 2014, Dr. deBethizy served as executive chairman of Contera Pharma ApS until it was sold to Bukwang Pharma, and from July 2015 to November 2017, he served as chairman of Rigontec GmbH until it was sold to Merck Inc. He previously served as chairman of the boards of directors of Albumedix Ltd (sold to Sartorius AG in September 2022), Saniona AB, and TME Pharma NV and AG. Dr. deBethizy was also a member of the boards of directors of Asceneuron SA, Serendex Pharmaceuticals A/S, Enbiotix Inc., Ligocyte Pharmaceuticals until it was sold to Takeda Pharmaceutical Co Ltd, Biosource Inc., and NOXXON Pharma N.V. Dr. deBethizy has held adjunct appointments at Wake Forest University Babcock School of Management, Wake Forest University School of Medicine, and Duke University. Dr. deBethizy holds a Bachelor’s of Science in biology from the University of Maryland, College Park and a Master’s of Science and a Ph.D. in toxicology from Utah State University. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 159 |
| Dr. Pamela Klein Dr. Pamela Klein has served as a member of our Board of Directors since April 2016. Since 2008, Dr. Klein has been a principal and founder of PMK BioResearch, a company offering strategic consulting in oncology drug development to corporate boards, management teams and the investment community. She has also been a venture partner in Ysios Capital Partners, SGIEC, S.A.U. since 2023. She currently serves as a member of the board of directors of several companies including I-Mab and Patrys Ltd; as well as various scientific advisor boards. In 2023, Dr. Klein also joined the boards of directors of Frontier Medicines Corp, Ona Therapeutics SL, and Sardona Therapeutics, Inc. Previously, Dr. Klein served on the board of directors of FStar Therapeutics, Inc. until March 2023, Jiya Acquisition Corp, and Spring Bank Pharmaceuticals, Inc. until its merger with F-Star Therapeutics in July 2020. Dr. Klein previously spent seven years at the National Cancer Institute as research director of the NCI-Navy Breast Center, after which she joined Genentech as vice president of development until 2001. She also served as chief medical officer for Intellikine, Inc., which was acquired by Takeda American Holdings. Dr. Klein holds a Bachelor’s degree in biology from California State University and an M.D. from Stritch School of Medicine, Loyola University Chicago and is trained in internal medicine and medical oncology. Anthony A. Rosenberg Anthony A. Rosenberg has served as a member of our Board of Directors since April 2017. He currently serves as CEO of TR Advisory Services GmbH, his own consultancy firm advising on business development, licensing, and mergers and acquisitions. Mr. Rosenberg also currently serves as chairman of the boards of directors of Oculis SA and Cullinan Oncology. Previously Mr. Rosenberg held the positions of Managing Director at MPM Capital, a venture capital firm (2015 until 2020); head of M&A and Licensing of Novartis International (2013 to 2015); and head of business development and licensing at Novartis Pharma (2005 to 2012). Mr. Rosenberg also previously served on the boards of directors of SiO2 Material Science (until March 2023), Radius Health Inc., TriNetX, Inc., iOmx Therapeutics AG, and Clinical Ink, Msc. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 160 |
| Mr. Rosenberg has a Bachelor of Science with honors from the University of Leicester and a Master’s of Science in physiology from the University of London. James M. Daly James M. Daly has served as a member of our Board of Directors since May 2018. Mr. Daly currently also serves as a director of Acadia Pharmaceuticals, Inc., Bellicum Pharmaceuticals, Inc., and Madrigal Pharmaceuticals, Inc. He was formerly a member of the board of Chimerix, Inc. and Halozyme. In 1985, he joined GlaxoSmithKline where he held various positions, including senior vice president of the respiratory division with full responsibility for sales, marketing and medical affairs. Mr. Daly moved to Amgen in 2002 where he was senior vice president for the North America commercial operations until 2011. In 2012, he joined Incyte Corp, a publicly-traded company focused on oncology and inflammation, where he was chief commercial officer until June 2015. Mr. Daly holds a Bachelor’s of Science and an MBA from the University at Buffalo, State University of New York. Camilla Sylvest Camilla Sylvest has served as a member of our Board of Directors since September 2022. Ms. Sylvest currently serves as the executive vice president of commercial strategy and corporate affairs of Novo Nordisk A/S. Ms. Sylvest has more than 27 years of working experience within Novo Nordisk A/S and was based in Switzerland, Denmark, Germany, Malaysia, and Mainland China. Over the years, Ms. Sylvest has headed up Novo Nordisk A/S affiliates of growing size and complexity in Europe. She was also corporate vice president of the business area Oceania and Southeast Asia and senior vice president and general manager of the Novo Nordisk A/S region of Mainland China. Ms. Sylvest also serves as the vice chair of Danish Crown A/S. Ms. Sylvest holds a Master’s of Science in economics from the University of Southern Denmark and an executive MBA from the Scandinavian Management Institute. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 161 |
| Ana Cespedes Ana Cespedes has served as a member of our Board of Directors since December 2022. Ms. Cespedes is the chief operating officer of the International AIDS Vaccine Initiative (IAVI), a global organization dedicated to developing accessible vaccines and antibodies for infectious diseases. Prior to joining IAVI, Ms. Cespedes held several roles at Merck KGaA, most recently serving as global head of strategy and engagement, government, and public affairs. She founded and led the global market access and pricing function for the company and worked with stakeholders to communicate the clinical, economic, and societal value of innovative medicines. Prior to that, Ms. Cespedes led the first integrated corporate affairs group at Serono Iberia and Merck Spain, was managing director of the Spanish branch of the company’s nonprofit organization, and worked as a senior consultant at Arthur Andersen. Ms. Cespedes is a founding member of the National Congress of Corporate Affairs in Spain, the London School of Economics Market Access Academy, and the Cooperation for Oncology Data. She is also the founder of Living Mindfulness S.L. Ms. Cespedes is also a member of the steering committee of ProPatiens Institute. Ms. Cespedes holds a Bachelor’s of Pharmacy and a PhD from the Complutense University of Madrid, a Master in General Management (PDG) from IESE Business School and an Executive Certificate on Strategy and Innovation from the Massachusetts Institute of Technology. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 162 |
| The following table sets forth certain information with respect to the current non-executive members of our Board of Directors, including their ages, as at December 31, 2023: Name Age Gender Position Nationality Date of Initial Appointment Date of last (re- ) Appointment Term Expiration Peter K. M. Verhaeghe 65 M Non-Executive Director (chairperson) Belgium October 15, 2008 May 10, 2022 2026 Werner Lanthaler1) 55 M Non-Executive Director (vice-chairperson) Austria July 9, 2014 May 10, 2022 2024 Steve Krognes1) 55 M Non-Executive Director U.S. and Norway February 27, 2023 February 27, 2023 2027 J. Donald deBethizy 73 M Non-Executive Director U.S. May 13, 2015 May 2, 2023 2025 Pamela Klein 62 F Non-Executive Director U.S. April 28, 2016 May 12, 2020 2024 Anthony A. Rosenberg 70 M Non-Executive Director UK April 26, 2017 May 11, 2021 2025 James M. Daly 62 M Non-Executive Director U.S. May 8, 2018 May 10, 2022 2026 Camilla Sylvest 51 F Non-Executive Director Denmark September 8, 2022 September 8, 2022 2026 Ana Cespedes 50 F Non-Executive Director Spain December 12, 2022 December 12, 2022 2026 1) Werner Lanthaler resigned effective February 27, 2023 and was succeeded by Steve Krognes effective February 27, 2023. The address for our non-executive directors is our registered office, Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 163 |
| The following table sets forth the companies and partnerships of which the current non-executive members of our Board of Directors have been a member of the administrative, management or supervisory bodies or partner at any time in the previous five years, indicating whether or not the individual is still a member of the administrative, management or supervisory bodies or partner, as of the date of this Annual Report, other than argenx or our subsidiaries: Name Current Past Peter K. M. Verhaeghe VVGB Advocaten – Avocats PharmaNeuroBoost NV Biocartis SA Participatiemaatschappij Vlaanderen NV Fujirebio Europe NV (formerly Innogenetics NV) miDiagnostics NV Tibotec-Virco NV Merisant France SAS Merisant Company 2 sàrl CzechPak Manufacturing s. r. o. Bever Zwerfsport BV Bioqube Factory Fund I NV Haretis SA Werner Lanthaler1) Bioxell SpA Pantec Biosolutions AG AC Immune SA Evotec SE J. Donald deBethizy White City Consulting ApS Rigotec GmbH Newron Pharmaceuticals SpA TME Pharma NV and AG Protteris, Inc. Saniona AB Lophora ApS Albumedix A/S Asceneuron SA Albumin Holdings ApS Innovent LLC Pamela Klein Olema Oncology Jiya Acquisition Corp. PMK BioResearch Patrys Limited I-Mab F-Star Therapeutics, Inc. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 164 |
| Name Current Past Anthony A. Rosenberg Cullinan Oncology Inc. Radius Health, Inc. Oculis SA TriNetX, Inc. Clinical Ink, Inc. iOmx Therapeutics AG MPM Capital SiO2 Material Science TR Advisory Services GmbH James M. Daly Acadia Pharmaceuticals Inc. Chimerix, Inc. Halozyme Therapeutics, Inc. Coherus Biosciences Bellicum Pharmaceuticals, Inc. Madrigal Pharmaceuticals Camilla Sylvest Novo Nordisk World Diabetes Foundation Crown A/S Ana Cespedes International AIDS Vaccine Initiative (IAVI) Instituto ProPatients Merck KGaA Merck Spain Serono Iberia Arthur Andersen Steve Krognes Denali Therapeutics Inc. R/S Global Guardant Health, Inc. Corvus Pharmaceuticals Inc. Gritstone bio Inc. ClavystBio 1) Werner Lanthaler resigned effective February 27, 2023 and was succeeded by Steve Krognes effective February 27, 2023. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Directors | 165 |
| Senior Management Our senior management team acts as our executive management. Of these people, only our CEO, Mr. Tim Van Hauwermeiren, is part of our Board of Directors as executive director. Our senior management team comprised of the following persons in 2023 and on the date of this Annual Report (appointment/retirement dates noted as relevant). Tim Van Hauwermeiren Tim Van Hauwermeiren co-founded our Company in 2008 and has served as our CEO since July 2008. He has served as a member of our Board of Directors since July 2014. Mr. Van Hauwermeiren has more than 20 years of general management and business development experience across the life sciences and consumer goods sectors. He also serves on the boards of directors of iTeos Therapeutics, Inc. and RayzeBio, Inc. Mr. Van Hauwermeiren holds a Bachelor’s of Science and Master’s of Science in bioengineering from Ghent University and an executive MBA from the Vlerick School of Management. Keith Woods Keith Woods served as our chief operating officer from April 2018 to March 2023, at which time he was succeeded by Karen Massey. Mr. Woods transitioned to serve as an advisor to our Board of Directors. He has over 30 years of experience in the biopharmaceutical industry. Mr. Woods most recently served as senior vice president of North American operations for Alexion Pharmaceuticals, Inc. (Alexion). Within Alexion, he previously served as vice president and managing director of Alexion UK, overseeing all aspects of Alexion’s UK business, and as vice president of U.S. operations and executive director of sales. Prior to joining Alexion, Mr. Woods held various positions of increasing responsibility within Roche, Amgen, and Eisai Co., Ltd. over a span of 20 years. He holds a Bachelor’s of Science in marketing from Florida State University. 3.2.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Senior Management | 166 |
| Karen Massey (effective March 13, 2023) Karen Massey has served as our chief operating officer since March 2023. Ms. Massey has over 20 years of experience in the pharmaceutical and biotechnology industry, including in commercial, product development, corporate strategy, and innovation roles. Prior to joining argenx, Ms. Massey was with Genentech (Roche Group) for over nine years, where she most recently served as senior vice president of product development and global clinical operations and previously held various commercial leadership roles across marketing and business operations, including as the vice president of the multiple sclerosis and neuromyelitis optica business. Ms. Massey started her biopharmaceutical career in marketing at Pfizer Inc., and returned there, after two years as a management consultant at Bain & Company, to take on leadership positions in corporate strategy and sales and as a commercial lead in Latin America. Ms. Massey holds a Bachelor’s of Economics from the University of Sydney and an MBA from the NYU Stern School of Business. Karl Gubitz Karl Gubitz has served as our chief financial officer since June 2021. Mr. Gubitz previously worked at Pfizer Inc. for nearly 20 years, most recently as vice president of finance within the global oncology business. With-in Pfizer Inc., Mr. Gubitz held country, regional, and global positions, and consistently delivered top-line growth. He managed teams of over 250 colleagues in financial leadership roles within the global internal medicine and global innovative products businesses. Prior to joining Pfizer Inc. in 2003, Mr. Gubitz held various management roles at PricewaterhouseCoopers LLP. Mr. Gubitz holds an MBA from Henley Management College, a Bachelor’s degree in computing from the University of South Africa, and Bachelor’s of Commerce from the University of Pretoria. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Senior Management | 167 |
| Dr. Peter Ulrichts Peter Ulrichts has served as our chief scientific officer since January 2023. In this role, he oversees the development of all clinical and pre-clinical compounds within our pipeline. Dr. Ulrichts previously served in various roles at the Company since he joined us in 2010, including, most recently, as our head of clinical science. As a research scientist, Dr. Ulrichts was involved in the development of various therapeutic antibodies for the treatment of cancer and autoimmune diseases. In 2013, he headed the development of our FcRn antagonist efgartigimod until the first-in-human clinical trial. He subsequently transitioned to become the lead scientist of our efgartigimod program. Dr. Ulrichts holds a Bachelor’s of Science in chemistry from Katholieke Universiteit Leuven, as well as a Master’s degree in Biotechnology and a Ph.D. in Biomedical Sciences, both from Ghent University. Malini Moorthy Malini Moorthy has served as our general counsel since February 2022. She has over 25 years of extensive global legal and compliance experience in the biopharmaceutical and medical device industries. She was most recently senior vice president and chief deputy general counsel of legal, compliance, and government affairs at Medtronic plc, where she played a pivotal role in shaping and driving enterprise and functional strategies. Before joining Medtronic plc, Ms. Moorthy spent four years at Bayer Corporation as the head of global litigation and investigations and 10 years at Pfizer Inc. , where she progressed to lead civil litigation globally. Ms. Moorthy began her career as a law firm associate, first with McCarthy Tétrault LLP and Genest Murray Desbrisay Lamek LLP in Toronto, Canada and then Salans LLP (now Dentons US LLP) in New York City. She holds a Bachelor of Arts in political science and economics from the University of North Carolina at Chapel Hill and a Bachelor of Laws from the Faculty of Law at Queen’s University in Canada. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Senior Management | 168 |
| Luc Truyen Luc Truyen has served as our chief medical officer since April 2022 and previously served as our head of research and development operations management from September 2021 to April 2022. Prior to this, Dr. Truyen was with Johnson & Johnson (and its subsidiary companies) for over 20 years holding various leadership positions, primarily within neuroscience. In his most recent position prior to joining argenx, Dr. Truyen was global head of development and external affairs for neuroscience, managing the strategy and delivery of the early and late portfolio of assets for mood disorders, schizophrenia, and neurodegenerative and neuroinflammatory disorders. Besides Dr. Truyen’s strong track record in clinical development resulting in several globally innovative drug approvals, his broad-based experience also includes leading global clinical development operations for the whole Johnson & Johnson pharmaceutical group as well as serving as the head of research and development and chief medical officer of Janssen Alzheimer Immunotherapy Research & Development LLC, an internal spin-out from Johnson & Johnson. Dr. Truyen holds an M.D. and Ph.D. in Neurology from the University of Antwerp. Arjen Lemmen Arjen Lemmen joined argenx in 2016 and has served as our vice president of corporate development & strategy since 2019. He has successfully executed several transactions including a number of programs within the IIP. Prior to joining the Company, Mr. Lemmen served as a corporate finance specialist at Kempen & Co NV focusing on mergers and acquisitions, equity capital markets and strategic advisory transactions in the European life sciences industry. He holds a Bachelor of Science in life science & technology from the University of Groningen and a Master of engineering management from Duke University. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Senior Management | 169 |
| Andria Wilk Andria Wilk joined argenx as global head of quality in 2020. Ms. Wilk has more than 20 years of experience in quality assurance (QA) within the pharmaceutical industry. Most recently, Ms. Wilk served as senior director, head of medical, regulatory & clinical QA (MRC QA) at H Lundbeck A/S (Lundbeck), where she managed the global MRC QA group based in the EU, US, and Asia. In this role, she was responsible for the global audit programs and QA support for all clinical trial and post-marketing activities and related computerized systems. Prior to Lundbeck, she held various QA positions of increasing responsibility within AstraZeneca PLC, Takeda Global Research, Development Centre Europe, and Astellas Pharma Inc. Ms. Wilk holds a joint Bachelor’s of Science in pharmacology and biochemistry, is a member of the Research Quality Association and observing board member of The European Forum for Good Clinical Practices. The following table sets forth certain information with respect to the members of our senior management, including their ages, as of December 31, 2023: Name Age Postion Nationality Date of Initial Appointment Tim Van Hauwermeiren 51 CEO and Executive Director Belgium July 15, 2008 Keith Woods1) 56 Chief Operating Officer U.S. April 5, 2018 Karen Massey1) 45 Chief Operating Officer Australia March 13, 2023 Karl Gubitz 54 Chief Financial Officer South Africa June 1, 2021 Peter Ulrichts 44 Chief Scientific Officer Belgium January 1, 2023 Malini Moorthy 54 General Counsel Canada February 14, 2022 Arjen Lemmen 39 Vice-President Corporate Development & Strategy The Netherlands May 1, 2016 Andria Wilk 51 Global Head of Quality UK January 13, 2020 Luc Truyen 59 Chief Medical Officer Belgium April 1, 2022 1) Keith Woods retired as COO effective March 13, 2023 and was succeeded by Karen Massey effective March 13, 2023. The address for our senior management is Industriepark-Zwijnaarde 7, 9052 Zwijnaarde (Ghent), Belgium. The following table sets forth the companies and partnerships of which the members of our senior management (or persons who have been members of our senior management in 2023) have been a member of the administrative, management or supervisory bodies or partner at any time in the previous five years, indicating whether or not the individual is still a member of ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Senior Management | 170 |
| the administrative, management or supervisory bodies or partner, as of the date of this Annual Report, other than argenx or our subsidiaries: Name Current Past Tim Van Hauwermeiren Iteos Therapeutics, Inc. Aelin Therapeutics NV RayzeBio, Inc Keith Woods1) X-4 Pharmaceuticals – Neurogene Inc T-Scan Therapeutics Rocket Pharma Karen Massey1) – Genentech, Inc. Karl Gubitz – Pfizer Inc. Peter Ulrichts – – Malini Moorthy – Medtronic plc Arjen Lemmen OncoVerity – Andria Wilk European Forum for Good Clinical Practice (EFGCP) H Lundbeck A/S Luc Truyen – Johnson & Johnson 1) Keith Woods retired as COO effective March 13, 2023 and was succeeded by Karen Massey effective March 13, 2023. Conflict-of-Interest Transactions Directors must immediately report any (potential) direct or indirect personal interest in a matter that conflicts with the interests of the Company and the business connected with it to the chairperson of our Board of Directors and to the other directors. Directors must also provide all relevant information, including information concerning their spouse, registered partner or other partner, foster child and relatives by blood or marriage up to the second degree as defined in the DCC. The non-executive directors will decide, without the director concerned being present, whether there is a conflict of interest. Under Dutch requirements, a conflict of interest in relation to a director in any event exists if the Company intends to enter into a transaction with a legal entity (i) in which such director personally has a material financial interest, (ii) which has an executive director or a member of the management board who is related under family law to such director or (iii) in which such director has an executive or non-executive position. A director will not participate in any discussions and decision making if he or she has a conflict of interest in the matter being discussed. In case because of this no resolution can be adopted by the executive directors, the non-executive directors will resolve on the matter. All transactions in which there are conflicts of interest with directors will be agreed on terms that are customary in the sector concerned. Decisions to enter into transactions in which there are conflicts of interest with directors that are of material significance to us or to the relevant director require the approval of the non-executive directors. All transactions between the Company and legal or natural persons who hold at least one tenth of our shares will be agreed on terms that are customary in the sector in which we and our combined businesses are active. The non-executive directors are required to approve such transactions that are of a material significance to us or to such persons. 3.2.6 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Conflict-of-Interest Transactions | 171 |
| Dutch law provides that transactions with related parties are material and thereby require approval of the Board of Directors if they are (a) not entered into in the ordinary course of our business or (b) not concluded on normal market terms. The Board of Directors has established an internal procedure to periodically assess whether transactions are concluded in the ordinary course of business and on normal market terms. Material transactions must be made public by the Company at the time the transaction is entered into. Transactions with related parties are considered material if (i) information on the transaction qualifies as inside information under the (Regulation (EU) No. 596/2014) (MAR) and (ii) such transaction is entered into with one or more holders of shares in the Company representing at least 10% of issued share capital, or a member of our Board of Directors. Transactions that are individually non-material, but which are entered into with the same related party during the same fiscal year, must be evaluated in the aggregate to determine if they are material. There are no arrangements or understandings in place with major shareholders, customers, suppliers or others pursuant to which any member of our Board of Directors or senior management team has been appointed. There are no conflicts of interests between the Company and any administrative, management and supervisory bodies and senior management, nor are there any potential conflicts of interests of the members of our Board of Directors and senior management between any duties to the Company and their private interests and or other duties. In connection with our initial U.S. public offering, we entered into a related party transaction policy. Code of Business Conduct and Ethics We adopted a Code of Business Conduct and Ethics (Code of Conduct), that is applicable to all of our employees and directors. The Code of Conduct is available on our website at www.argenx.com/investors/governance/rules-codes-compliance. The audit and compliance committee of our Board of Directors is responsible for overseeing the Code of Conduct and is required to approve any waivers of the Code of Conduct for employees and directors. We expect that any amendments to the Code of Conduct, and any waivers of its requirements, will be disclosed on our website. Report of the Non-Executive Directors Meetings Our Board of Directors had five formal meetings in the course of 2023. The meetings were held in the months February, May, July, October and December. The committees of the Board of Directors also convened regularly and at least once per quarter (please refer to sections “Report Audit and Compliance Committee” to “Report Commercialization Committee” below for the separate reports of the committees). All Board of Director meetings and all formal committee meetings were also attended by Mr. Van Hauwermeiren, as executive director. In addition, several members of the senior management team were invited to discuss specific items included on the Board of Director and committee meetings’ agendas. 3.2.7 3.3 3.3.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Code of Business Conduct and Ethics | 172 |
| Attendance Record Board of Director Meetings In 2023, five Board of Directors meetings were held. The meeting attendance rate for our directors is set out in the table below. Name Number of meetings attended in 2023 since appointment (and up to resignation, as applicable) Attendance % Peter K. M. Verhaeghe (chairperson) 5 100% Tim Van Hauwermeiren 5 100% Werner Lanthaler1) 1 100% Steve Krognes1) 4 100% J. Donald deBethizy 5 100% Pamela Klein 5 100% Anthony A. Rosenberg 5 100% James M. Daly 5 100% Camilla Sylvest 5 100% Ana Cespedes 5 100% 1) Werner Lanthaler resigned effective February 27, 2023 and was succeeded by Steve Krognes effective February 27, 2023. In 2023, all of the five Board of Directors meetings with solely the non-executive directors being present were held as closed sessions at the beginning or the end of other meetings. These meetings were attended by all non-executive directors appointed at such time. Name Number of meetings attended in 2023 since appointment Attendance % Peter K. M. Verhaeghe (chairperson) 5 100% Werner Lanthaler 5 100% J. Donald deBethizy 5 100% Pamela Klein 5 100% Anthony A. Rosenberg 5 100% James M. Daly 5 100% Camilla Sylvest 5 100% Ana Cespedes 5 100% Activities The agenda for the Board of Directors centers around the key business objectives for long-term value creation and the key risks involved, as well as the manner in which the senior management team implements our strategy including our research and development pipeline and the commercialization of our products, our culture to ensure proper monitoring by the non-executive directors, our financial position and finance readiness as well as the results of our subsidiaries, significant investment proposals, yearly budgets, the internal risk 3.3.2 3.3.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Report of the Non-Executive Directors | 173 |
| management and control system, diversity, equity and inclusion, succession planning and remuneration and appointment matters. In 2023, specific attention was given to the statutory and governance topics including the long-term succession and contingency planning of the Board of Directors and senior management, leading to the appointment of Mr. Steve Krognes as non-executive director and chair of the audit and compliance committee and the renewal of the appointment of Mr. J. Donald deBethizy as non-executive director and his appointment as vice-chair of the Board of Directors. The Board of Directors furthermore discussed the long-term succession planning of the senior management team leading to the appointment of Ms. Karen Massey as our chief operating officer. The Board of Directors discussed the review and approval of forecasts, the Company’s product portfolios, business and corporate development, cybersecurity landscape, review and approval of consolidated financial statements, update research and developments, committee reports, financing of the Company and the approval of the proposed agendas and other meeting documents for our General Meeting, among other things. Board Evaluation The Board of Directors evaluates its functioning and the functioning of its committees and of each individual director annually. The evaluation process is performed with the help of an external professional board evaluation consultant. In 2023, the evaluation was performed by Nasdaq Governance Solutions. The evaluation includes preparing specific questionnaires focusing on the skills and competences most relevant to us, and the most material board topics and challenges we face. The written questionnaire is then followed up by one-to-one interviews with the representative of Nasdaq Governance Solutions with each member of the Board of Directors, followed by a debrief and discussion held with the external evaluator and the entire Board of Directors both in writing (in form of a report) and in the form of a live discussion of the evaluation report aimed at distilling specific learnings and conclusions. Based on the self-evaluation performed, the non-executive directors concluded that the Board of Directors and its committees had properly discharged their responsibilities during 2023. The Board of Directors identified certain strengths and weaknesses and adopted a plan for further board development and succession in 2024. In general non-executive directors appreciate the high effectiveness of the Board and the functioning of its committees and consider that (i) the Board is high functioning, committed, open, transparent and very engaged and (ii) the Board committees are strong and work well. Report Audit and Compliance Committee The audit and compliance committee reports regularly to our Board of Directors on the exercise of its functions. It informs our Board of Directors about all areas in which action or improvement is necessary in its opinion and produces recommendations concerning the necessary steps that need to be taken. The audit review and the reporting on that review cover the Company and its subsidiaries as a whole. In 2023, the main points of discussion at the meetings were the 2022 consolidated financial statements and press release as well as interim consolidated financial statements and press releases, internal audit and external auditors’ reports, the, the review of quarterly forecasts, updates on tax priorities, compliance, cash management, CSRD readiness, the company’s ethics and compliance program, the company’s cyber security program and the company’s privacy program. 3.3.4 3.3.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Report of the Non-Executive Directors | 174 |
| 2023年,召開了5次審計和合規委員會會議。我們董事的會議出席率 如下表所示。 姓名 自 預約出席率 以來在2023年出席的會議次數 Peter K.M.Verhaeghe 5 100% Werner Lanthaler 1)1 100% Steve Krognes(主席)1)4 100% Anthony A.Rosenberg 5 100% James M.Daly 5 100% 1)Werner Lanthaler辭職,從2023年2月27日起由Steve Krognes接替2023. 報告薪酬和提名 委員會 薪酬和提名委員會協助董事會處理其他事項,包括定期審查我們的薪酬政策,準備薪酬 提案,定期評估董事會的規模和組成,以及制定高級管理團隊關於高級管理人員遴選標準和 任命程序的政策。在2023年的審議期間,主要的 討論主題是C級長期繼任規劃、股權薪酬 和持股指導方針、人才招聘、公司的追回政策我們的薪酬話語權投票結果以及在我們於2023年5月2日舉行的年度股東大會(2023年股東大會)上對薪酬話語權投票之前和之後與代理顧問和投資者的互動。 2023年,我們舉行了五次正式的薪酬和提名委員會會議。我們董事的會議出席率列於下表。 名稱 自 預約出席率 以來,2023年出席的會議數量 Peter K.M.Verhaeghe 5 100% Ana Cespedes 5 100% J.Donald de Bethizy(主席)5 100% 報告研究與開發委員會研發委員會的職能是向我們的研究和開發管理、一般管理和董事會發出意見,並監督我們的研發目標、戰略和措施。2023年,委員會舉行了五次正式會議,主要關注科學的願景和戰略,公司的研發渠道,包括臨牀前和臨牀階段的候選產品,其商業階段產品的潛在未來跡象以及與我們的國際投資協議有關的發展。 3.3.6 3.3.7 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 非執行董事2023年年度報告|175 |
| 我們董事的會議出席率如下表所示。 名稱 自2023年以來出席的會議數量 自 預約出席率% J.Donald de Bethizy 5 100% 帕梅拉·克萊因5 100% David·萊西(主席)5 100% 報告商業化委員會 商業化委員會的職能是為董事會提供品牌和非品牌戰略營銷計劃的意見板。2023年,委員會舉行了五次正式會議,主要集中在我們推出VYVGART的執行情況以及為未來可能推出的產品做準備,有待進一步批准。 我們董事的會議出席率列在下表中。 名稱 自 預約出席人數% 安東尼·A·羅森伯格5 100% 詹姆斯·M·戴利(主席)5 100% Camilla Sylvest 5 100% 薪酬報告和 薪酬聲明 代言薪酬和對薪酬政策的擬議修訂 介紹 迴應異議後,2023年出席的會議數量 股東在公司2022年和2023年年度股東大會上對薪酬話語權進行投票,我們與利益相關者、股東和代理顧問進行了廣泛接觸。這組利益相關者佔公司已發行股本的60%以上。這導致了一項修訂薪酬政策的提案, 預計將於2024年3月21日或前後以草案形式公佈(2024年薪酬政策草案),這需要在2024年5月7日舉行的公司年度股東大會(2024年股東大會)上獲得批准。鼓勵本報告的讀者閲讀《2024年薪酬政策草案》和相應的説明性説明,這兩份説明將在公司網站at https://www.argenx.com/investors/shareholder-meetings. Although上提供。以下不是對公司現行的2021年薪酬政策(2021年薪酬政策)擬議變化的詳盡摘要, 鼓勵您閲讀全文,包括隨附的説明性説明。公司 認為有必要提請您注意將在《2024年薪酬政策草案》中提出的以下主要變化。 3.3.8 3.4 3.4.1 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告薪酬報告|176 |
| Non-executive pay · Stock options will no longer be granted to non-executive directors · Non-executive pay will take the form of cash remuneration and equity remuneration in the form of RSUs · Non-executive RSU grants will have a vesting period of 1 year and a holding period of 3 years after vest and as such underlying shares cannot be sold until after 4 years from the grant date · Non-executive RSUs will be awarded based on a benchmarked target cash value, awarded in shares subject to the aforementioned holding requirements · Minimum holding requirements extending at least 2 years beyond term of service will continue to apply Executive equity incentives · Performance share units (PSUs) will be introduced in the executive compensation plan, attaching financial and non-financial performance conditions to the vesting of the PSUs · A significant portion of short term variable pay will be linked to financial targets · PSU performance conditions will link for at least 50% of their target value to financial targets · Non-financial targets will relate to measurable sustainable long term value creating outcomes linked to the Company’s key value drivers: ‘innovation and pipeline development’ and ‘people and culture’ · PSUs will not vest prior to the third anniversary of the grant date and only to the extent applicable performance conditions are met · The target equity pay opportunities for the CEO, chief financial officer (CFO) and COO (the NEOs) will continue to be set between the 50th percentile and 75th percentile of the reference group and will in any case not exceed 15x base cash compensation · All equity grants will be subject to multi-year (at least 3 years) vesting periods and/or holding requirements · Minimum holding requirements extending at least 2 years beyond term of service will continue to apply Executive short term cash incentive · Short term cash incentives will be linked to multiple strategically relevant targets, which, in turn, will be linked to clearly measurable outcomes · At least 50% of short term variable pay will be linked to financial targets · The target cash pay opportunity (target and maximum), measurement and evaluation and pay-out will be disclosed · Considering the rapid growth and development of the Company and the environment in which it operates, discretionary adjustment of the total variable pay within the set limits by the Board of Directors will be possible, but in the event this happens, a clear and detailed explanation of the use of such discretion will be included in the Company’s remuneration report The principles above will be applicable for remuneration granted and targets set after the approval of the Draft 2024 Remuneration Policy, which requires a majority vote of more than 75% at the 2024 General Meeting. If such majority is not achieved, the Company will, in accordance with Dutch law, be obliged to continue to apply the 2021 Remuneration Policy until a new policy gets approved at the 2024 General Meeting. You are encouraged to read this 2023 remuneration report in conjunction with the Draft 2024 Remuneration Policy and the ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Say-on-pay and proposed Amendments to the Remuneration Policy | 177 |
| accompanying explanatory notes. It is noted that this 2023 remuneration report describes the application of the Company’s 2021 Remuneration Policy for the fiscal year 2023. 2023 Remuneration Introduction The 2021 Remuneration Policy rewards contributions to achieving Company objectives and generating stakeholder value. The aim is to provide competitive remuneration packages that align with market practices in the key markets where the Company competes for talent. The Company conducts regular reviews (at least once every three years) of director and senior management members’ total remuneration (both in quantum and in program design) and makes comparisons against the Company’s reference companies. The 2021 Remuneration Policy and total compensation aligns or slightly exceeds the market median for fixed compensation, benefits, and short-term variable compensation. The long-term incentive component consists of equity grants, the size of which is positioned between the 50th and the 75th percentile of the global reference group. The 2021 Remuneration Policy was adopted at the 2021 General Meeting with a 76% majority vote and is available on the Company’s website at https://www.argenx.com/investors/governance/remuneration-policy . Reference group – general For the 2023 remuneration which was set following a benchmark exercise conducted in the August – September 2022 timeframe, the Company worked with an independent third party compensation advisor, AON Radford. The Company continued to benchmark against both US and European peer groups to account for being a global company competing for talent against European based and US based companies. The aim is to deliver globally competitive compensation supporting the execution of Company’s business strategy and aligning with long-term sustainable value creation for its stakeholders. The following criteria were used to select the reference group for the 2023 remuneration as part of the Company’s benchmark performed in the third quarter of 2022, ahead of setting the long term incentive schemes for 2023 in December 2023 and the annual cash compensation for 2023 in first quarter of 2023: · Sector: Biotech and Pharmaceuticals · Stage of development: Market · Market Capitalization: primary ~1/3x – 3x argenx’s 30‐day average market value as of 5/20/ 22, secondary $5‐50 billion · Headcount: primary ~1/3x – 3x the midpoint of argenx’s projected financial years ended 31 December, 2022 and 2023 headcounts, secondary 300‐2500 employees · Revenue: less than $1 billion revenues · Years public: less than 10 years since IPO With the goal of arriving at a sufficiently sized U.S. and EU peer group of companies disclosing detailed compensation information, a number of companies were added to the European peer group following a qualitative review by AON Radford to identify companies with relevant similarities in business model and therapeutic focus. This leads to the following selection of peer groups used by us in the 2022 benchmark for the 2023 compensation plans: Note: for completeness’ sake, this is not the peer group the Company used in 2023 for its 2024 remuneration. The 2023 benchmark takes into account ongoing discussions and insight on the development of the 2021 Remuneration Policy and plans, as well as stakeholder feedback received. On or around the date of this report, the peer group for the 2024 remuneration will 3.4.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 2023 Remuneration | 178 |
| be reported on the Company’s website at https://www.argenx.com/investors/governance/ remuneration-policy. Company Name Company Ticker Country of Trade Abcam Plc ABC GBR Acadia Healthcare Company, Inc. ACHC USA ALK‐Abelló A/S ALK.B DNK Alnylam Pharmaceuticals, Inc. ALNY USA Amicus Therapeutics, Inc. FOLD USA Ascendis Pharma A/S ASND USA BeiGene, Ltd. 6160 USA Biohaven Pharmaceutical Holding Company Ltd. BHVN USA BioMarin Pharmaceutical Inc. BMRN USA BioNTech SE BNTX USA Blueprint Medicines Corp BPMC USA CRISPR Therapeutics AG CRSP USA Denali Therapeutics Inc DNLI USA Evotec SE EVT DEU Galapagos NV GLPG NLD Genmab A/S GMAB DNK Hikma Pharmaceuticals Plc HIK GBR Horizon Therapeutics Public Limited Company HZNP USA Idorsia Ltd IDIA CHE Incyte Corporation INCY USA Intellia Therapeutics, Inc. NTLA USA Intra‐Cellular Therapies, Inc. ITCI USA Ionis Pharmaceuticals, Inc. IONS USA Mirati Therapeutics, Inc. MRTX USA Neurocrine Biosciences, Inc. NBIX USA Recordati SpA REC ITA Sarepta Therapeutics, Inc. SRPT USA Seagen Inc. SGEN USA Swedish Orphan Biovitrum AB SOBI SWE UCB SA UCB BEL uniQure N.V. QURE USA Vifor Pharma AG VIFN CHE ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 2023 Remuneration | 179 |
| 獎勵級別 董事會根據對標工作的結果,根據《2021年薪酬政策》確定獎勵級別,該政策在這方面包含以下框架: 非執行董事高級管理人員(包括首席執行官) 現金薪酬 全球 參考組中美國公司的第50個百分位數 美國公司在 美國高管參考小組中的第50個百分位數,和 美國EU-based executives Equity-based compensation 50th百分位數參考組中的歐盟公司的第75個百分位數或附近。 參考 組中的美國公司 第50至75個百分位數的公司 The Reference group ar Gr g oup enx Factors Risk Go Corporate 維納斯資本 分享財務 審核報表 財務非財務 信息 argenx年度報告2023 2023薪酬|180 |
| 本章詳細概述了2023年支付給以下近地天體的薪酬:首席執行官、首席財務官和首席運營官。在這些近地天體中,只有首席執行官是阿根克斯的法定董事。 近地天體2023年的薪酬包括基本工資、可變現金薪酬、公司股權和福利。 高管薪酬政策 大部分高管薪酬以浮動薪酬的形式提供,這是績效薪酬(短期現金獎勵、股票期權)和服務薪酬(RSU)的組合。可變(短期)薪酬允許董事會設定具有挑戰性的年度目標,使近地天體的優先事項與公司的短期戰略目標保持一致。 股票期權形式的公司股權激勵近地天體在股票期權的長期(3年)歸屬期內為公司(股票價格)增值做出貢獻。限制性股票單位形式的公司股權還為限制性股票單位的長期(4年)歸屬 期間創造價值提供了激勵。浮動薪酬、股票期權和RSU的組合確保了對短期專注於短期戰略目標和實現短期戰略目標的平衡激勵,同時有助於 可持續的長期價值創造,並確保高管的長期承諾(留任)。此外,該公司還提供市場標準的遣散費安排以及養老金和附帶福利, 包括按照比利時慣例發放的最高3,948歐元(4,266美元)的公司獎金。此外,根據DCGC,在確定高管的薪酬方案時,情景分析 每年進行一次,在確定短期和長期激勵計劃下的總薪酬水平和目標以及 最高獎勵時被考慮在內。 高管薪酬總額 下表列出了過去3年支付給近地天體的薪酬總額: (美元)基本工資1) 基本工資 與前一年相比 %1) 簽約 獎金 公司 獎金 可變短線 Br}期限 獎勵 可變現金 作為百分比的 最大 機會 以股票形式表示的補償 選項2) 以 形式表示的補償 其他 福利3) %固定 (總計)4)總計 CEO-Tim Van Hauwermeiren 2023 655,787 0%-590,215 60%8,084,6055)2,575,174 39,054 6%11,944,835 2022 638,910%-910%766,682 60%4,174,684 2,159,689 38,342 9%7,778,298 2021 651,986 5%-1,186 586,787 60%3,895,370 2,084,509 45,177 10%7,265,014 3.4.3 ar Gr g oup enx Factors Risk Go Corporate vernance資本 分享財務 評審報表 財務非財務 信息 《2023年年度報告》被任命為高管薪酬|181 |
| (in $) Base salary1) Base salary in % change vs the prior year1) Sign on bonus Corporate bonus Variable short term incentive Variable cash as % of maximum opportunity Compen-sation in the form of stock options2) Compen-sation in the form of RSUs Other benefits3) % fixed (of total)4) Total CFO – Karl Gubitz 2023 516,043 6% – 3,556 260,866 40% 2,626,062 1,287,587 62,798 12% 4,756,913 2022 487,600 79% – 3,745 243,800 40% 2,623,633 1,356,048 91,203 12% 4,806,030 2021 271,646 N/A6) – 2,235 108,659 40% 3,181,721 1,629,272 31,809 6% 5,225,342 COO – Karen Massey7) 2023 481,471 N/A 338,0008) 2,921 467,662 50% 3,939,093 2,296,517 127,393 8% 7,653,057 COO – Keith Woods9) 2023 305,022 -48% – – – – – – 46,034 100% 351,056 2022 583,774 5% – 3,745 583,774 50% 2,601,982 1,364,014 205,032 15% 5,342,321 2021 555,975 5% – 4,095 347,484 50% 2,430,402 1,316,532 116,041 14% 4,770,529 1) The base salary of the CEO is paid in EUR (for 2023 base salary exchange rate 1.0815 EUR/$ used in this table), the base salary of the COO is paid in CHF (for 2023 base salary exchange rate of 1.1135 EUR/CHF used in this table). The percentage presenting the change in salary is calculated using the currency of payment. 2) Amounts shown represent the expenses with respect to stock options measured using the Black Scholes formula. For a description of the assumptions used in valuing these awards, see note 13 “Share-based payments” to the consolidated financial statements. 3) Other benefits consists of the lease of a company car, employer-paid medical insurance premiums, pension contributions, social security costs and other allowances. 4) Fixed compensation is considered as Base salary and Other benefits. 5) Target pay level set in number of options and RSUs as part of benchmark performed in September of the prior year (target value $6,986,986, grant occurred on the first business day of July 2023. Share price increase between setting the grant using argenx’s 30-day average stock price of $366.58 as of July 22, 2022 and the share price of $389.73 at the date of grant) explains variation between target compensation level and the final calculation displayed in the table above. These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the Stock Options awards granted in 2023 measured using the Black Scholes formula with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiary versus non-Belgian beneficiary. The fair value of equity granted to Belgian beneficiaries was higher than that of non-Belgian beneficiaries resulting in Mr. Van Hauwermeiren’s stock based compensation expense to be higher than other beneficiaries. For a description of the assumptions used in valuing these awards, see note 13 “Share-based payments” to the consolidated financial statements. Further, see subsection equity to section “Named Executive Officer Remuneration”. 6) Karl Gubitz joined as CFO in June 2021, and consequently no comparison for base salary 2021 to 2020 is possible, as well as comparison for base salary 2022 to 2021 being distorted. 7) Karen Massey joined as COO in March 2023, and consequently no comparisons to 2022 and before were possible, and Ms. Massey’s remuneration shows the remuneration paid for the period March 13, 2023 through December 31, 2023. Her variable pay pay-out has been pro-rated to reflect this as well. 8) In 2023, the Company paid a sign-on bonus to Karen Massey to allow the Company to make an overall competitive offer of employment and in recognition of lost corporate benefits as a result of early departure at Ms. Massey’s previous employer. Ensuring a competitive offer in this way and securing Ms. Massey as the Company’s new COO was deemed by the Board of Directors to be in the best interest of the Company and its stakeholders. 9) Keith Woods resigned as COO March 2023 and his employment relationship ended on June 30, 2023 and consequently the remuneration numbers show his remuneration for the period January 1, 2023 through June 30, 2023. No equity award or variable pay was paid to Mr. Woods in the year ended December 31, 2023. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 182 |
| 與2022年相比,2023年,近地天體的基本工資與2022年相比,按照Argenx員工總體業績增長指導方針(CEO+0%,CFO+6%,COO於2023年加入)進行了增長。這些加薪是在對個人在上一年的表現進行了 審查之後(S),根據對 基準數據的綜合分析,基準數據顯示了基本工資相對於相關外部和內部同行的相對定位。此流程確保公司的薪酬方案公平反映個人績效,同時保持競爭力並與市場保持一致。適用的績效原則和基本工資增加框架與適用於組織中所有員工的相同,並以個人在上一期間的表現和貢獻為基礎。從2022年到2023年,我們的CEO 拒絕獲得基本工資增長。 關於CFO,董事會認可了2022年的傑出表現,包括 完成和超額完成短期目標,並確定CFO的薪酬低於同行評審的參考組CFO基本工資的中點。因此,為了與所有員工一致應用的薪酬實踐 ,首席財務官的基本工資增加了,績效增加和額外的加薪使首席財務官接近基準中點,與2022年相比,2023年的基本工資總計增加了6%。 可變現金 近地天體有資格獲得2023年預定短期業績目標的可變現金薪酬,目標可變現金薪酬設置為其基本工資的百分比(首席執行官為60%,首席運營官為50%,首席財務官為40%)。董事會為每個目標設定了200%的派息上限,總派息上限為200% 。董事會對每個目標的派息進行了評估,下表詳細説明瞭“達到目標”、“每個目標的最高派息”和“實際派息”。此外,如果總結果不能公平地代表績效薪酬,董事會有 調整支出的酌處權。如果使用這種自由裁量權,將在薪酬report. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx年度報告2023年指定高管薪酬|183中詳細説明 |
| 首席執行官 在考慮首席執行官的浮動薪酬時,董事會初步審查了公司2023年業務計劃的關鍵目標是否實現。這些主要目標是: 1.通過實現公司雄心勃勃的商業業務計劃,實現VYVGART的收入目標;為已確定的產品、候選產品和適應症以及 新創新不斷髮展和發展管道,為公司構建可持續的長期價值創造潛力: a.及時獲得VYVGART皮下批准 b.根據積極的試驗結果,在最短的時間內提交高質量的CIDP BLA;和 c.在管道中增加至少3個新的高度創新的計劃,擴展目標為5個超額完成);以及 iii.業務關鍵職能的成功繼任、招聘和入職(包括 董事會和首席運營官繼任的幾名新成員,以及公司各團隊新招聘的創紀錄數量的員工), 實現雄心勃勃的招聘計劃,並在 爆炸性增長期保護和提升公司文化;以及 ii.考慮到與計劃中的臨牀試驗讀數(CIDP、ITP、PV、MMN)相關的多個預期臨牀“關鍵時刻”,以及另一個商業執行的關鍵年份,首席執行官需要在 透明和平衡的溝通方面投入巨資,積極和持續地確保基於數據的預期和組織彈性,同時保持對公司執行能力的信任。這需要 在外部(與投資者的溝通)和作為公司高級管理層負責人的內部實現。 首席執行官的總目標實現導致目標派息的125%(詳細提供如下), 董事會利用其酌情權額外獎勵98,368美元(目標激勵的25%),以表彰 成功交付包括上述關鍵目標的公司業務計劃,並特別 認可商業發佈的持續成功,遠遠超出內部和外部 預期。董事會認為,獎勵首席執行官高質量的執行及其對公司可持續的長期價值創造軌跡的影響符合公司及其利益相關者的利益。 以下所述目標的實現,加上酌情向上調整,導致首席執行官總共獲得590,215美元的浮動薪酬,支付目標派息的150%和最高opportunity. ar Gr g oup enx Factors Risk Go Corporate vernance資本的75% 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告指定的高管薪酬|184 |
| Personal targets set for the CEO, in addition to his overall responsibility for delivering the business plan, were the following: Target Measurement (how the Board of Directors evaluated the target) % Target pay-out Max pay-out (in $) Achievement Actual pay-out (% of target) Actual pay-out (in $) Line up the next wave of immunology breakthroughs: at least 5 new highly innovative programs entered the pipeline Baseline: at least 3 new programs, Stretch: 5 or more 25 98,368 196,736 Overachieved Number of programs added significantly exceeded stretch target, warranting maximum pay-out of target. 200% 196,736 Proactively manage argenx’s clinical moments of truth · Build organizational resilience ahead of key clinical trial readouts · Ensure data based expectations internally and externally ahead of key clinical trial read-outs · Protect and enhance investors’ trust in argenx’s ability to execute External and internal trust in argenx’s ability to execute maintained, even in the context of some setbacks Support of key long term shareholders maintained Ability to attract and retain top talent preserved and/or enhanced 25 98,368 196,736 Achieved Continued support of key shareholders maintained, key talent retained and further key talent hired and onboarded, throughout significant wins (CIDP) and setbacks (ITP, PV) 100% 98,368 Future-proof company leadership, strengthen board effectiveness. Support successful board succession, maximally leverage the board as a resource Successful selection, hiring and onboarding of new COO Continued access to talent, knowledge and expertise of departing COO, CMO and (founder) CSO, if feasible Ensured excellent onboarding of new board talent, positioned new board members for maximum impact 25 98,368 196,736 Achieved Successful hiring and onboarding of top quality COO Retiring COO, CMO and founder CSO positioned for continued impact through long term Board of Directors’ committee advisory roles Successful onboarding of 3 new key Board of Directors’ positions 100% 98,368 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 185 |
| Target Measurement (how the Board of Directors evaluated the target) % Target pay-out Max pay-out (in $) Achievement Actual pay-out (% of target) Actual pay-out (in $) Execute the 2023 hiring plan, delivering on successful onboarding of a record number of new hires (including 2022 hires, integrate what argenx scaled) in support of the company’s execution ambitions. Safeguard and enhance the corporate culture and align the entire employee base behind the strategic priorities Company-wide understanding of and support for the business plan and alignment around top priorities 2023 hiring plan delivered Record number of new hires throughout 2022 and 2023 successfully onboarded and embraced the argenx cultural values Corporate culture protected, no critical talent departures, voluntary turnover remained stable 25 98,368 196,736 Achieved Company business plan delivered through exceptional cross functional and cross regional collaboration and commitment of all employees Voluntary turnover rates remained relatively stable (less than 1% deviation from 2022 number) and on the low end of market averages (4.27% (2022) to 5% (2023)) Broad participation (295 argonauts across regions and functions) participated in newly launched dedicated forum designed to protect and enhance argenx’s company cultural pillars 100% 98,368 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 186 |
| 首席財務官 在考慮首席財務官的浮動薪酬時,董事會主要審查了首席財務官在2023年負有主要責任的公司業務計劃的以下主要目標是否實現了 。這些主要目標是: i.實現VYVGART的收入目標(伸展目標‘根據年度運營計劃的收入顯著超過目標); ii.考慮到VYVGART最近的推出以及在競爭激烈的環境中面臨的持續挑戰, 主動並持續地確保基於數據的外部對財務業績的預期,同時保持對公司執行能力的信任;以及 ii.審計與合規委員會新任主席和 的成功交接和入職繼續保持強勁的審計業績(內部和外部)。 為首席財務官設定的個人目標如下: 目標 衡量 (董事會如何評估目標)% Target pay-out Max pay-out (in$)業績 實際 支出 (目標的百分比) 實際 派息 (以美元為單位) 以優惠條件籌集至少5億美元的資本,為公司 提高雄心水平和 相應的業務計劃提供資金 業績:至少以優惠條件籌集5億美元 延伸:以 優惠條件籌集7.5億美元以上 25 52,173 104,346美元,超額完成 10億+競爭條件籌資, 納斯達克(當時)歷史上最大的生物技術後續融資, 修訂後的業務計劃資金充足 200%104,346確保圍繞efgartigimod發佈的外部和內部預期保持一致 財務業績基本一致 或略高於市場預期 繼續支持和留住關鍵股東和關鍵人才 繼續鞏固公司在透明度和可靠性方面的聲譽 25 52,173 104,346實現了 四個季度的‘節拍和提升’活動 內部和外部預期之間沒有明顯差距 100%52,173Ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年報任命高管薪酬|187 |
| 目標 衡量 (董事會如何評估目標)% Target pay-out Max pay-out (in$)業績 實際支出 (目標的百分比) 實際支出 (單位:$) 簡化和改進整個公司的財務 規劃流程,儘可能簡化, 顯著減少非財務人員在財務規劃上花費的時間 流程 顯著簡化了整個公司的財務 規劃和管理流程 減少了幹擾,更專注於創新 25 52,173 104,346實現了 更廣泛的公司領導層 公認Argenx財務規劃 流程是同類最佳的,提供了 簡化的財務規劃流程 ,結果出色允許 團隊專注於其核心職責,同時受益於高質量的財務規劃 100%52,173保護和保留公司和 關鍵資產,進一步建立與審計和合規委員會的合作關係,並確保成功 新的審計和合規委員會主席,支持高質量的內部和外部審計 流程,確保卓越的 透明度 確保審計和合規委員會的新主席 出色上任,委員會定位為最大影響 25 52,173 104,346實現了 成功入職新審計 和合規委員會主席,促進了與內部和外部審計員良好的工作關係 ,高質量的 流程和高水平的透明度導致了乾淨的審計 結果 100%52,173 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 Argenx 2023年年度報告命名為高管薪酬|188 |
| 首席運營官 在考慮首席運營官的浮動派息時,董事會初步審查了公司2023年業務計劃的主要商業和運營目標是否實現。這些關鍵的 目標是: i.新的首席運營官快速而成功地上崗,將自己定位為具有高度影響力,並設計她的 多年戰略計劃; i.實現VYVGART的收入目標;以及 III。執行業務關鍵(商業)職能的繼任、招聘和入職,包括在新的 區域,實施雄心勃勃的招聘計劃,同時通過爆炸性增長時期保護和提升公司文化。 為首席運營官設定的個人目標,以及她對提供商業業績的總體責任 ,以下是: 目標 衡量 (董事會如何評估目標)% Target pay-out Max pay-out (in$)業績 實際支出 (目標的百分比) 實際支出 (單位:美元) 完成年度運營計劃目標 商業收入 目標:未披露 擴展:超額完成目標至少 10% 35 109,121 218,242超額完成營收20億美元, 顯著高於內部和外部預期 僅在美國就有超過10億美元的收入 4個季度節拍和提升活動 200%218,242負責地構建了Argenx在關鍵非美國地區的全球 擴張計劃,填補了關鍵職位 非美國地區業務計劃的關鍵方面交付了 15 46,766 93,532個超額完成的業務計劃中與非美國商業擴張相關的擴展目標 ,包括成功進入加拿大和首次銷售,成功 執行關鍵分銷 合作伙伴關係強勁的業務案例 為新地區打造,在德國、意大利、西班牙獲得顯著成功 在中國成功啟動 200%93,532 ar Gr g oup enx Factors Risk Go Corporate vernance資本 分享財務 審核報表 財務非財務 信息 argenx 2023年年報任命高管薪酬|189 |
| 目標 衡量 (董事會如何評估目標)% Target pay-out Max pay-out (in$)成就 實際 支出 (目標的百分比) 實際 支出 (單位:$) 實施商業運營模式 以實現始終如一的卓越發布, 反映Argenx的文化和價值觀 為商業發佈設計的高影響力運營模式 制定了組織設計 ,這為我們建立了長期的商業成功 25 77,944 155,887實現了 商業運營模式的成功內部重組, 建立了跨職能指示和 現場團隊,完全符合公司的文化支柱,同時 顯著超額完成收入 目標 在加入後立即超出預期,在全球範圍內迅速建立真正的信任和 支持,並贏得商業(和現場)團隊的完全信任和支持,使首席運營官 獲得長期成功和 組織影響力 100%77,944制定並獲得董事會對argenx 2030商業 戰略的批准,確定關鍵戰略選項 和投資情景 引人注目的2030遠景由管理團隊的廣泛參與設計,並得到董事會的認可 25 77,944 155,887實現了 為未來價值創造建立了高質量的多年商業戰略 ,使管理團隊和董事會 協調了這項戰略計劃任務2030, 審查了計劃,經 董事會審核通過 100%77,944 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 argenx 2023年年度報告任命高管薪酬|190 |
| Corporate bonus All employees are eligible to annually earn a corporate bonus with a maximum of €3,948 ($4,266) per year, based on Company-wide goals. In 2023, the targets focused on (i) simplifying high-impact cross-functional processes, (ii) saving more on an undisclosed dollar amount in negotiated spend to advance financial responsibility and (iii) increased cybersecurity awareness. In 2023, the corporate bonus was achieved by 83.34% and a corresponding pay-out of €3,291/ $3,556 was made to all employees. Equity In 2023, the Company granted a mix of stock options and RSUs to the NEOs. The number of instruments to be granted in the course of 2023 were determined pursuant to the benchmark exercise performed with the help of AON Radford in September of 2022, where the equity compensation levels of CEO, CFO and COO roles within the Company’s reference group were reviewed. The target values for long term incentives were then converted into a number of stock options and a number of RSUs to be granted, using a Black Scholes valuation of $151,03 per stock option and a value of $366,58 per RSU, based on a 30-day average stock price used for the August 2022 benchmark. This number of equity instruments was then embedded into the equity allocation scheme for 2023. It is noted that as a result of the method of fixing the number of instruments based on the benchmark value and the time in between the benchmark and the grant, the value of the grant as ultimately reported will differ from the target value if the stock price has changed (positively or negatively) between the date of fixing the allocation scheme and the date of the grant. More specifically, if the stock price increases between date of setting the allocation scheme and the grant date, the Black Scholes value of the stock options will increase, assuming all other parameters stay stable. The Company is taking concrete steps to close the time gap between the benchmark and the grant date, as will be further explained in the Draft 2024 Remuneration Policy and accompanying explanatory notes. Specifically for the COO, the Board of Directors decided to grant equity in excess of the base numbers for the COO role, as a means to attract the new COO in a highly competitive talent market. The Board of Directors deemed enabling Ms. Massey to join the Company of crucial importance for the Company’s long term succession planning. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 191 |
| The following table sets out the number, value and key terms of equity instruments granted to the NEOs in 2023: RSUs granted in 2023 Stock options granted in 2023 Name # RSUs Key terms Value at grant in $ Benchmark value in $ # Stock options Exercise price in € Exercise price in $ Key terms Value at grant1) in $ Benchmark value1) in $ Total Tim Van Hauwermeiren – CEO 6,700 RSUs vest and are settled in 4 equal instalments of 25% over a 4 year period. 2,575,174 2,456,086 30,000 355.40 387.35 1/3 vests after year 1 2/3 vest in monthly instalments in year 2 and 3 Options not exercisable until the 4th calendar year after the grant year 8,084,6052) 4,530,900 10,659,779 Karl Gubitz – CFO 3,350 1,287,587 1,228,043 15,000 355.40 387.35 1/3 vests after year 1 2/3 vest in monthly instalments in year 2 and 3 2,626,062 2,265,450 3,913,649 Karen Massey – COO 5,025 1,931,380 1,842,065 22,500 355.40 387.35 3,939,093 3,398,175 5,870,474 Sign-on grant 950 Vested on the date of the grant 365,137 348,251 – – – – – – 365,137 Keith Woods – COO – – – – – – – – – – 1) Target pay level set in number of stock options and RSUs as part of benchmark performed in September of the prior year (target value $6,986,986, grant occurred on the first business day of July 2023), share price increase between setting the grant using argenx’s 30-day average stock price of $366.58 as of July 22, 2022 and the share price of $389.73 at the date of grant explains variation between target compensation level and the final calculation displayed in the table above. These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the stock options awards granted in 2023 measured using the Black Scholes formula with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiaries versus non-Belgian beneficiaries. The fair value of equity granted to Belgian beneficiaries was higher than that of non-Belgian beneficiaries resulting in Mr. Van Hauwermeiren’s stock based compensation expense to be higher than other beneficiaries. For a description of the assumptions used in valuing these awards, see Note 13 “Share-based payments” in our consolidated financial statements. 2) The reason that this amount is more than 2x the amount of the CFO, even though the number of equity instruments is exactly 2x that of the CFO, is due to different assumptions used in valuation applicable for Belgian based employees than for US based employees. Please see footnote 1) for further details. The table below shows (i) the stock options held as of January 1, 2023, (ii) the stock options granted to the NEOs which vested during the year ended December 31, 2023, (iii) the number of stock options scheduled to vest in the years ending December 31, 2024, December 31, 2025 and December 31, 2026 and (iv) the respective exercise price of such stock options. Each stock option was granted pursuant to the Equity Incentive Plan: ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 192 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specifi-cation plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Tim Van Hauwermeiren, CEO Equity incentive plan 14/12/2017– 01/12/2020 14/12/ 2017 (1) 31/12/ 2020 01/01/2021– 14/12/2027 21.17 72,500 – 72,500 – – – – – – 21/12/2018– 01/12/2021 21/12/ 2018 (1) 31/12/ 2021 01/01/2022– 21/12/2028 86.32 80,000 – – – – – – 80,000 – 20/12/2019– 01/12/2022 20/12/ 2019 (1) 31/12/ 2022 01/01/2023– 20/12/2029 135.75 80,000 – – – – – – 80,000 – 21/12/2020– 01/12/2023 21/12/ 2020 (1) 31/12/ 2023 01/01/2024– 21/12/2030 247.60 50,000 – – – 16,667 – – 50,000 – 24/12/2021– 01/12/2024 24/12/ 2021 (1) 31/12/ 2024 01/01/2025– 24/12/2031 309.20 25,000 – – – 8,334 8,333 8,333 25,000 25,000 23/12/2022– 01/12/2025 23/12/ 2022 (1) 31/12/ 2025 01/01/2026– 23/12/2032 359.60 25,000 – – – 8,333 16,667 16,667 25,000 25,000 03/07/2023– 01/07/2026 03/07/ 2023 (1) 31/12/ 2026 01/01/2027– 03/07/2033 355.40 – 30,000 – – – 30,000 30,000 30,000 30,000 Total 332,500 30,000 72,500 – 33,334 55,000 55,000 290,000 80,000 Karl Gubitz, CFO Equity incentive plan 01/07/2021– 01/07/2024 01/07/ 2021 (1) N/A 01/07/2022– 01/07/2031 255.10 24,000 – – – 8,000 4,667 4,667 24,000 – 01/07/2022– 01/07/2025 01/07/ 2022 (1) N/A 01/07/2023– 01/07/2032 357.50 16,000 – – – 7,556 8,444 8,444 16,000 – 03/07/2023– 01/07/2026 03/07/ 2023 (1) N/A 03/07/2024– 03/07/2033 355.40 15,000 – – – 15,000 15,000 15,000 – Total 40,000 15,000 – – 15,556 28,111 28,111 55,000 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 193 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specifi-cation plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Karen Massey, COO Equity incentive plan 03/07/2023– 01/07/2026 03/07/ 2023 (1) N/A 03/07/2024– 03/07/2033 355.40 – 22,500 – – – – 22,500 22,500 – Total – 22,500 – – – – 22,500 22,500 – Keith Woods, former COO Equity incentive plan 20/12/2019– 01/12/2022 20/12/ 2019 (1) N/A 20/12/2020– 20/12/2029 135.75 35,000 – 35,000 – – – – – – 21/12/2020– 30/6/2023 21/12/ 2020 (1) N/A 21/12/2021– 21/12/2030 247.60 50,000 – – – 16,667 – – 50,000 – 24/12/2021– 30/6/2023 24/12/ 2021 (1) N/A 24/12/2022– 31/03/2025 309.20 16,000 – – – 10,667 – – 16,000 – 23/12/2022– 30/6/2023 23/12/ 2022 (1) N/A 23/12/2023– 31/03/2026 359.60 16,000 – – 10,667 5,333 – – 5,333 – Total 117,000 – 35,000 10,667 32,667 – – 71,333 – 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd vest in equal instalments (24 in total) over the next two years, each time upon the 1st day of each next month. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 194 |
| 下表顯示了(I)截至2023年1月1日持有的RSU,(Ii)在截至2023年12月31日的年度內授予歸屬 的近地天體的RSU數量,以及(Iii)計劃在截至2024年12月31日、2025年12月31日、2026年12月31日和2027年12月31日的年度歸屬的RSU數量。根據股權激勵計劃授予每個RSU: 有關報告的財政年度的信息 期末餘額 期末餘額 董事姓名,職位績效期間獎勵日期 歸屬 日期 結束 保留期 在the year RSUs awarded RSUs forfeited RSUs vested RSUs subject開始時 持有的service condition RSUs awarded and unvested RSUs 在 持有的 關閉 年 RSU 受 保留期 Tim van Hauwermeiren,CEO 24/12/2021-24/12/2021(1)N/A 4,275--1,425-2,850 2,850- 23/12/2022-23/12/2026 23/12/2022(1)N/A 5,700--1,425-4,275 4,275- 03/07/2023-03/07/2023(1)N/A-6,700--6,700 6,700- 總計9,975 6,700-2,850-13,825,825- CFO 01/07/2021-01/07/2025 01/07/2021(1)N/A 4,050--1,350-2,700 2,700- 01/07/2022-01/07/2026 01/07/2022(1)N/A 3,600--900-2,700 2,700- 03/07/2023-03/07/2027 03/07/2023(1)N/A-3,350--3,350 3,350- 總計7,650 3,350-2,250-8,750 8,750- ,COO 03/07/2023-03/07/2027 03/07/2023(1)N/A-5,025-5,025,025- N/A 03/07/2023(2)N/A-950-950 950- 總計-5,975--5,975 5,975- 基思·伍茲,前首席運營官24/12/2021-30/06/2023 24/12/2021(1)N/A 2,700--2,700- 23/12/2022-30/06/2023 23/12/2022(1)N/A 3,600-2,700- 總計6,300-2,700 3,600- 1)RSU在四年內歸屬,並在授予之日的每個週年日歸屬總授予金額的四分之一。 2)RSU在grant. ar Gr g oup enx Factors Risk Go Corporate vernance資本之日歸屬 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告指定的高管薪酬|195 |
| 高管持股要求 2023年,公司對其高管團隊實施了以下持股要求: ·首席執行官:3倍基本工資 ·其他近地天體:1倍基本工資 最低股本必須在最長五年內建立,並在 任職期間和之後兩年內繼續適用。 養老金和附帶福利 支付給近地天體的福利取決於司法管轄區。對於首席執行官來説,這些福利包括比利時市場上的慣例福利,這些福利是比利時員工薪酬的標準組成部分:養老金 繳費、住院保險、代表津貼和公司汽車。對於首席財務官來説,這些 包括美國市場慣例的福利,是我們在美國的員工薪酬的標準組成部分:一家公司管理健康和401K計劃,公司匹配4%。對於首席運營官, 這些福利包括瑞士市場的慣例福利,是基於瑞士的員工福利的標準組成部分:汽車津貼、午餐津貼、醫療保險津貼、代表權津貼和養老金繳款。 根據我們的2021年薪酬政策,首席執行官有18個月的通知期(或 另選,12個月的遣散費代替通知)。對於我們的其他近地天體,沒有關於遣散費的合同安排。 在2023財年,沒有向NEOs. ar Gr g oup enx Factors Risk Go Corporate vernance資本支付遣散費 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告命名為高管薪酬|196 |
| Treatment of leaver equity With respect to Keith Woods, the Board of Directors determined his long-term equity incentives vested in full on June 30, 2023, consistent with the terms of his employment contract and a separate agreement made between him and the Company in which Mr. Woods agreed to stay on with the Company as long as necessary to identify, recruit and onboard a suitable replacement and to continue to contribute to long term value creation for the Company as a member of the Commercialization Committee (all as set out in a service agreement entered into between us and Mr. Woods, and for which no remuneration shall be paid): · all unvested stock options and RSUs granted prior to 2022 to and held by Mr. Woods vested on June 30, 2023, whereby Mr. Woods shall not be allowed to exercise stock options of which the vesting was accelerated pursuant to this resolution, or sell shares received pursuant to the settlement of RSUs of which the vesting was accelerated pursuant to this resolution, earlier than on the date on which such equity would normally have vested in accordance with the rules of the applicable argenx equity plan (assuming normal continuation of vesting in the situation where Mr. Woods would not have retired from the company). The sole exception to the aforementioned exercise/sell restriction shall be the sale of equity to the extent solely needed to cover tax liabilities directly following from the aforementioned accelerated vesting and/or settlement of equity; and · equity granted to Mr. Woods in 2022 vested only through the first anniversary of the grant date and the remainder was forfeited per December 31, 2023. Claw back policy In the event that any variable remuneration (cash or equity) is paid to members of senior management, including the NEOs, based on financial information which later proves to be incorrect and leads to an accounting restatement (i) due to the material noncompliance of the Company with any financial reporting requirement under applicable securities laws, including any required accounting restatement to correct an error in previously issued financial statements of the Company that is material to the previously issued financial statements of the Company, or (ii) that corrects an error that is not material to previously issued financial statements of the company, but would result in a material misstatement if the error were corrected in the current period or left uncorrected in the current period, then the difference between the paid compensation and the compensation which would have been payable without such accounting restatement, shall be claimed back from the executive, all as further set out in the Executive Compensation Clawback Policy, as adopted by the Board of Directors on July 25, 2023. In fiscal year 2023, no variable remuneration was clawed back and no variable remuneration was adjusted (retroactively). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Named Executive Officer Remuneration | 197 |
| Remuneration of Other Senior Management Members For the purposes of the equity reporting by the Company under European legislation, all senior level employees reporting directly to the CEO qualify as the Company’s ‘senior management members’, and for the purposes U.S. governance reporting requirements, as the Company’s ‘executives’. For that reason and in compliance with U.S. disclosure requirements, the remuneration disclosures in relation to this more extensive group of senior personnel (excluding the NEOs) in this remuneration report is presented on an aggregated basis, with the exception of equity remuneration, which presented on an individual basis. Aggregate compensation for other senior management members The following table sets forth information regarding aggregate compensation paid to members of the senior management (other than the NEOs) during fiscal year ended December 31, 2023. (in $) Compensation Base salary 2,202,303 Corporate bonus 17,790 Variable short term incentive 1,134,786 Compensation in the form of stock options 13,333,334 Compensation in the form of RSUs 5,534,702 Other benefits1) 882,154 Total 23,105,069 1) Other benefits consists of the lease of a company car, employer-paid medical insurance premiums, pension contributions, social security costs and allowances. Equity for other senior management members granted in 2023 The following table sets forth information regarding stock option and RSU awards granted to members of the senior management during fiscal year ended December 31, 2023: 3.4.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 198 |
| RSUs granted in 2023 Stock options granted in 2023 Name # RSUs Key terms Value at grant in $ # Stock options Exercise price in € Exercise price in $ Key terms Value at grant1) in $ Total Peter Ulrichts 3,350 RSUs vest and are settled in 4 equal instalments of 25% over a 4 year period. 1,287,586.88 15,000 355.40 387.35 1/3 vests after year 1 2/3 vest in monthly instalments in year 2 and 3 3,420,785 4,708,372 Malini Moorthy 3,350 1,287,586.88 15,000 355.40 387.35 2,626,062 3,913,649 Luc Truyen 3,350 1,287,586.88 15,000 355.40 387.35 3,420,785 4,708,372 Arjen Lemmen 3,350 1,287,586.88 15,000 355.40 387.35 2,626,062 3,913,649 Andria Wilk 1,000 384,354.29 4,600 355.40 387.35 1,239,639 1,623,994 1) These amounts do not reflect the actual economic value realized by the beneficiary. Amounts shown represent the expenses with respect to the Stock options awards granted in 2023 measured using the Black Scholes formula with unobservable assumptions. The assumptions used in the fair valuation differ between Belgian beneficiaries versus non-Belgian beneficiaries. The fair value of equity granted to Belgian beneficiaries was higher than that of non-Belgian beneficiaries resulting in stock based compensation expense to be higher for Belgian beneficiaries than other beneficiaries. For a description of the assumptions used in valuing these awards, see note 13 to our consolidated financial statements in section “Consolidated Financial Statements”. The table below shows (i) the stock options held as of January 1, 2023, (ii) the stock options granted to members of senior management (other than the NEOs) which vested during the year ended December 31, 2023, (iii) the number of stock options scheduled to vest in the years ending December 31, 2024, December 31, 2025 and December 31, 2026 and (iv) the respective exercise price of such stock options. Each stock option was granted pursuant to the Equity Incentive Plan: Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specifi-cation plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Peter Ulrichts, CSO Equity incentive plan 28/06/2018– 01/06/2021 28/06/ 2018 (1) 31/12/ 2021 01/01/2022– 28/06/2023 80.82 750 – 750 – – – – – – 21/12/2018– 01/12/2021 21/12/ 2018 (1) 31/12/ 2021 01/01/2022– 21/12/2023 86.32 5,250 – 5,250 – – – – – – 20/12/2019– 01/12/2022 20/12/ 2019 (1) 31/12/ 2022 01/01/2023– 20/12/2029 135.75 12,870 – 7,870 – – – – 5,000 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 199 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specifi-cation plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period 21/12/2020– 01/12/2023 21/12/ 2020 (1) 31/12/ 2023 01/01/2024– 21/12/2030 247.60 9,900 – – – 2,550 – – 9,900 – 24/12/2021– 01/12/2024 24/12/ 2021 (1) 31/12/ 2024 01/01/2025– 24/12/2026 309.20 3,420 – – – 1,140 1,140 1,140 3,420 3,420 23/12/2022– 01/12/2025 23/12/ 2022 (1) 31/12/ 2025 01/01/2026– 23/12/2027 359.60 16,000 – – – 8,377 7,623 7,623 16,000 16,000 03/07/2023– 01/07/2026 03/07/ 2023 (1) 31/12/ 2026 01/01/2027– 03/07/2028 355.40 15,000 – – – 15,000 15,000 15,000 15,000 Total 48,190 15,000 13,870 – 12,067 23,763 23,763 49,320 34,420 Malini Moorthy, Legal Counsel Equity incentive plan 01/04/2022– 01/04/2025 01/04/ 2022 (1) N/A 01/04/2023– 01/04/2032 282.50 24,000 – 7,500 – 13,333 10,667 10,667 16,500 – 03/07/2023– 01/07/2026 03/07/ 2023 (1) N/A 03/07/2024– 03/07/2033 355.40 – 15,000 – – – 15,000 15,000 15,000 – Total 24,000 15,000 7,500 – 13,333 25,667 25,667 31,500 – Luc Truyen, CMO Equity incentive plan 01/10/2021– 01/10/2024 01/10/ 2021 (1) 31/12/ 2024 01/01/2025– 01/10/2026 259.50 24,000 – – – 8,000 6,667 6,667 24,000 24,000 23/12/2022– 01/12/2025 23/12/ 2022 (1) 31/12/ 2025 01/01/2026– 23/12/2027 359.60 16,000 – – – 5,333 10,667 10,667 16,000 16,000 03/07/2023– 01/07/2026 03/07/ 2023 (1) 31/12/ 2026 01/01/2027– 03/07/2028 355.40 – 15,000 – – 15,000 15,000 15,000 15,000 Total 40,000 15,000 – – 13,333 32,334 32,334 55,000 55,000 Arjen Lemmen, Vice President of Corporate Equity incentive plan 28/06/2018– 01/06/2021 28/06/ 2018 (1) 31/12/ 2021 01/01/2022– 28/06/2028 80.82 695 – – – – – – 695 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 200 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Specifi-cation plan Performance period Award date Vesting date End of retention period Exercise period Exercise price of stock option in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options forfeited Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Development & Strategy 21/12/2018– 01/12/2021 21/12/ 2018 (1) 31/12/ 2021 01/01/2022– 21/12/2028 86.32 15,952 – – – – – – 15,952 – 20/12/2019– 01/12/2022 20/12/ 2019 (1) 31/12/ 2022 01/01/2023– 20/12/2029 135.75 50,000 – 12,445 – – – – 37,555 – 21/12/2020– 01/12/2023 21/12/ 2020 (1) 31/12/ 2023 01/01/2024– 21/12/2030 247.60 50,000 – – – 16,667 – – 50,000 – 24/12/2021– 01/12/2024 24/12/ 2021 (1) 31/12/ 2024 01/01/2025– 24/12/2031 309.20 16,000 – – – 5,334 5,333 5,333 16,000 16,000 23/12/2022– 01/12/2025 23/12/ 2022 (1) N/A 23/12/2023– 23/12/2032 359.60 16,000 – – – 5,333 10,667 10,667 16,000 16,000 03/07/2023– 01/07/2026 03/07/ 2023 (1) N/A 03/07/2024– 03/07/2033 355.40 – 15,000 – – – 15,000 15,000 15,000 15,000 Total 148,647 15,000 12,445 – 27,334 31,000 31,000 151,202 47,000 Andria Wilk, Global Head of Quality Equity incentive plan 20/12/2019– 01/12/2022 20/12/ 2019 (1) 31/12/ 2022 01/01/2023– 20/12/2024 135.75 9,400 – 9,400 – – – – – – 21/12/2020– 01/12/2023 21/12/ 2020 (1) 31/12/ 2023 01/01/2024– 21/12/2025 247.60 9,900 – – – 2,662 – – 9,900 – 24/12/2021– 01/12/2024 24/12/ 2021 (1) 31/12/ 2024 01/01/2025– 24/12/2031 309.20 4,446 – – – 757 756 756 4,446 4,446 23/12/2022– 01/12/2025 23/12/ 2022 (1) 31/12/ 2025 01/01/2026– 23/12/2027 359.60 4,600 – – – 2,347 2,253 2,253 4,600 4,600 03/07/2023– 01/07/2026 03/07/ 2023 (1) 31/12/ 2026 01/01/2027– 03/07/2033 355.40 – 4,600 – – 770 3,830 4,600 4,600 3,830 Total 28,346 4,600 9,400 – 6,536 6,839 7,609 23,546 12,876 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd during the following two years vest in equal instalments (24 in total) over the next two years, each time upon the 1st day of each next month. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 201 |
| The table below shows (i) the RSUs held as of January 1, 2023, (ii) the RSUs granted to members of senior management (other than the NEOs) which vested during the year ended December 31, 2023 and (iii) the number of RSUs scheduled to vest in the years ending December 31, 2024, December 31, 2025, December 31, 2026 and December 31, 2027. Each RSU was granted pursuant to the Equity Incentive Plan: Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Performance period Award date Vesting date End of retention period RSUs held at the beginning of the year RSUs awarded RSUs forfeited RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year RSUs subject to a reten-tion period Peter Ulrichts, CSO 24/12/2021–24/12/2025 24/12/2021 (1) N/A 570 – – 190 – 380 380 – 23/12/2022–23/12/2026 23/12/2022 (1) N/A 3,600 – – 900 – 2,700 2,700 – 03/07/2023–03/07/2027 03/07/2023 (1) N/A – 3,350 – – – 3,350 3,350 – Total 4,170 3,350 – 1,090 – 6,430 6,430 – Malini Moorthy, Legal Counsel 01/04/2022–01/04/2026 01/04/2022 (1) N/A 5,400 – – 1,350 – 4,050 4,050 – 03/07/2023–03/07/2027 03/07/2023 (1) N/A – 3,350 – – – 3,350 3,350 – Total 5,400 3,350 – 1,350 – 7,400 7,400 – Luc Truyen, CMO 01/10/2021–01/10/2025 01/10/2021 (1) N/A 4,050 – – 1,350 – 2,700 2,700 – 23/12/2022–23/12/2026 23/12/2022 (1) N/A 3,600 – – 900 – 2,700 2,700 – 03/07/2023–03/07/2027 03/07/2023 (1) N/A – 3,350 – – – 3,350 3,350 – Total 7,650 3,350 – 2,250 – 8,750 8,750 – Arjen Lemmen, Vice President of Corporate Development & Strategy 24/12/2021–24/12/2025 24/12/2021 (1) N/A 2,700 – – 900 – 1,800 1,800 – 23/12/2022–23/12/2026 23/12/2022 (1) N/A 3,600 – – 900 – 2,700 2,700 – 03/07/2023–03/07/2027 03/07/2023 (1) N/A – 3,350 – – – 3,350 3,350 – Total 6,300 3,350 – 1,800 – 7,850 7,850 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 202 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Directors, Position Performance period Award date Vesting date End of retention period RSUs held at the beginning of the year RSUs awarded RSUs forfeited RSUs vested RSUs subject to a service condition RSUs awarded and unvested RSUs held at the closing of the year RSUs subject to a reten-tion period Andria Wilk, Global Head of Quality 24/12/2021–24/12/2025 24/12/2021 (1) N/A 741 – – 247 – 494 494 – 23/12/2022–23/12/2026 23/12/2022 (1) N/A 1,000 – – 250 – 750 750 – 03/07/2023–03/07/2027 03/07/2023 (1) N/A – 1,000 – – – 1,000 1,000 – Total 1,741 1,000 – 497 – 2,244 2,244 – 1) RSUs vest over a period of four years with 1/4th of the total grant vesting at each anniversary of the date of grant. 2) RSUs are vested at date of the grant. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Remuneration of Other Senior Management Members | 203 |
| Non-Executive Remuneration In accordance with the 2021 Remuneration Policy, the remuneration of the non-executive directors consists of (i) a fixed fee calculated on the basis of their membership or chairmanship of the Board of Directors and/or its committees and (ii) a long term equity incentive in the form of stock options and RSUs. It is noted that as part of the changes proposed for the 2021 Remuneration Policy, subject to its approval at the 2024 General Meeting, the Company will no longer compensate non-executive directors with stock options, but only in the form of cash and RSUs please refer to section 3.4.1 ‘Say-on-pay and proposed Amendments to the 2021 Remuneration Policy’. Total non-executive remuneration The following table sets forth the information regarding the compensation earned by the non-executive directors during fiscal year ended December 31, 2023: Name Fees earned or paid in cash (in $) Stock option awards (in $) RSU awards (in $) Total (in $) Peter K.M. Verhaeghe 94,629 431,179 134,524 660,332 Werner Lanthaler 11,716 – – 11,716 Steve Krognes 64,438 377,772 193,440 635,649 Pamela Klein 56,777 280,113 134,524 471,414 J. Donald deBethizy 67,592 280,113 134,524 482,229 Anthony A. Rosenberg 62,185 280,113 134,524 476,822 James M. Daly 67,592 280,113 134,524 482,229 Camilla Sylvest 54,073 210,085 101,085 365,244 Ana Cespedes 54,073 140,057 67,262 261,392 3.4.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 204 |
| Annual cash compensation The Board of Directors has set the annual base remuneration, the annual remuneration for members of the audit and compliance committee, the research and development committee, the remuneration and nomination committee and the commercialization committee and, in each case, the additional remuneration for the respective chairperson as follows: in $ Relevant body Position Fees in € Fees in $ Peter K.M. Verhaeghe Werner Lanthaler1) Steve Krognes1) Pamela Klein J. Donald deBethizy Anthony A. Rosenberg James M. Daly Camilla Sylvest Ana Cespedes Board of Directors Chairperson 75,000 81,110 81,110 – – – – – – – – Member 45,000 48,666 – 8,111 44,611 48,666 48,666 48,666 48,666 48,666 48,666 Audit & Compliance committee Chairperson 15,000 16,222 – 2,704 14,870 – – – – – – Member 7,500 8,111 8,111 – – – – 8,111 8,111 – – Remuneration & Nomination committee Chairperson 10,000 10,815 – – – – 10,815 – – – – Member 5,000 5,407 5,407 901 4,957 – – – – – 5,407 Commercialization committee Chairperson 10,000 10,815 – – – – – – 10,815 – – Member 5,000 5,407 – – – – – 5,407 – 5,407 – Research & Development committee Chairperson 15,000 16,222 – – – – – – – – – Member 7,500 8,111 – – – 8,111 8,111 – – – – Total 94,629 11,716 64,438 56,777 67,592 62,185 67,592 54,073 54,073 1) Resigned from Board of Directors following the board meeting of February 28, 2023 upon appointment and onboarding of Mr. Krognes. Compared to 2022, no changes were in 2023 made to the levels of cash compensation for the non-executive directors. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 205 |
| Equity compensation In 2023, in accordance with the 2021 Remuneration Policy, the non-executive directors received grants of stock options and RSUs, as follows: RSUs granted in 2023 Stock options granted in 2023 Name # RSUs Key terms Value at grant in $ # Stock options Exercise price in € Exercise price in $ Key terms Value at grant in $ Total Peter K.M. Verhaeghe 350 RSUs vest and are settled in 4 equal instalments of 25% over a 4 year period 134,524 1,600 355.40 387.35 Vesting upon third anniversary of the grant 431,179 565,703 Werner Lanthaler – – – – – – – Steve Krognes 525 193,440 2,400 355.40 387.35 377,772 571,212 Pamela Klein 350 134,524 1,600 355.40 387.35 280,113 414,637 J. Donald deBethizy 350 134,524 1,600 355.40 387.35 280,113 414,637 Anthony A. Rosenberg 350 134,524 1,600 355.40 387.35 280,113 414,637 James M. Daly 350 134,524 1,600 355.40 387.35 280,113 414,637 Camilla Sylvest 263 101,085 1,200 355.40 387.35 210,085 311,170 Ana Cespedes 175 67,262 800 355.40 387.35 140,057 207,319 The table below shows (i) the stock options held at January 1, 2023, (ii) the stock options granted to the non-executive directors which have vested during the year ended December 31, 2023, (iii) the number of stock options scheduled to vest in the years ending December 31, 2024, December 31, 2025 and December 31, 2026 and (iv) the respective exercise price of such stock options: Information regarding the reported financial year Opening balance During the Year Closing balance Name of Board member Perfor-mance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Peter K.M. Verhaeghe 30/09/2014– 30/09/2017 30/09/2014 (1) 31/12/2017 01/01/2018– 30/09/2024 3.95 1,969 – 1,969 – – – – – 30/09/2014– 30/09/2017 30/09/2014 (1) 31/12/2017 01/01/2018– 30/09/2024 2.44 2,885 – 2,885 – – – – – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 206 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Board member Perfor-mance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period 18/12/2014– 18/12/2017 18/12/2014 (1) 31/12/2017 01/01/2018– 18/12/2024 7.17 5,000 – 3,000 – – – 2,000 – 18/06/2016– 18/06/2019 18/06/2016 (1) 31/12/2019 01/01/2020– 18/06/2026 11.38 10,000 – – – – – 10,000 – 21/12/2018– 21/12/2021 21/12/2018 (1) 31/12/2021 01/01/2022– 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019– 20/12/2022 20/12/2019 (1) 31/12/2022 01/01/2023– 20/12/2029 135.75 10,000 – – – – – 10,000 – 21/12/2020– 21/12/2023 21/12/2020 (1) 31/12/2023 01/01/2024– 21/12/2030 247.60 10,000 – – 3,333 – – 10,000 – 24/12/2021– 24/12/2024 24/12/2021 (2) 31/12/2024 01/01/2025– 24/12/2031 309.20 2,700 – – – – 2,700 2,700 2,700 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 01/01/2026– 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 01/01/2027– 03/07/2033 355.40 – 1,600 – – – 1,600 1,600 1,600 Total 55,254 1,600 7,854 3,333 – 7,000 49,000 7,000 Werner Lanthaler 21/12/2018– 21/12/2021 21/12/2018 (1) N/A 21/12/2019– 21/12/2028 86.32 10,000 – 10,000 – – – – – 20/12/2019– 20/12/2022 20/12/2019 (1) N/A 20/12/2020– 20/12/2029 135.75 5,580 – 5,580 – – – – – 21/12/2020– 21/12/2023 21/12/2020 (1) N/A 21/12/2021– 21/12/2030 247.60 10,000 – 1,126 3,333 – – 8,874 – 24/12/2021– 24/12/2024 24/12/2021 (2) 01/12/2024 24/12/2022– 24/12/2031 309.20 2,700 – – – – – 2,700 – Total 28,280 – 16,706 3,333 – – 11,574 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 207 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Board member Perfor-mance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period Steve Krognes 03/04/2023– 03/04/2026 03/04/2023 (2) 31/12/2026 03/04/2024– 03/04/2033 340.70 – 2,400 – – – 2,400 2,400 2,400 Total – 2,400 – – – 2,400 2,400 2,400 Pamela Klein 18/06/2015– 18/06/2018 18/06/2015 (1) N/A 18/06/2016– 18/06/2025 11.44 – – – – – – – – 18/06/2016– 18/06/2019 18/06/2016 (1) N/A 18/06/2017– 18/06/2026 11.38 – – – – – – – – 21/12/2018– 21/12/2021 21/12/2018 (1) N/A 21/12/2019– 21/12/2028 86.32 10,000 – 8,500 – – – 1,500 – 20/12/2019– 20/12/2022 20/12/2019 (1) N/A 20/12/2020– 20/12/2029 135.75 10,000 – – – – – 10,000 – 21/12/2020– 21/12/2023 21/12/2020 (1) N/A 21/12/2021– 21/12/2030 247.60 10,000 – – 3,333 – – 10,000 – 24/12/2021– 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022– 24/12/2031 309.20 2,700 – – – – 2,700 2,700 2,700 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023– 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 1,600 – – – 1,600 1,600 1,600 Total 35,400 1,600 8,500 3,333 – 7,000 28,500 7,000 J. Donald deBethizy 18/06/2016– 18/06/2019 18/06/2016 (1) N/A 18/06/2017– 18/06/2026 11.38 10,000 – – – – – 10,000 – 21/12/2018– 21/12/2021 21/12/2018 (1) N/A 21/12/2019– 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019– 20/12/2022 20/12/2019 (1) N/A 20/12/2020– 20/12/2029 135.75 10,000 – – – – – 10,000 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 208 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Board member Perfor-mance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period 21/12/2020– 21/12/2023 21/12/2020 (1) N/A 21/12/2021– 21/12/2030 247.60 10,000 – – 3,333 – – 10,000 – 24/12/2021– 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022– 24/12/2031 309.20 2,700 – – – – 2,700 2,700 2,700 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023– 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 1,600 – – – 1,600 1,600 1,600 Total 45,400 1,600 – 3,333 – 7,000 47,000 7,000 Anthony A. Rosenberg 13/12/2016– 13/12/2019 13/12/2016 (1) N/A 13/12/2017– 13/12/2026 14.13 15,000 – – – – – 15,000 – 21/12/2018– 21/12/2021 21/12/2018 (1) N/A 21/12/2019– 21/12/2028 86.32 10,000 – – – – – 10,000 – 20/12/2019– 20/12/2022 20/12/2019 (1) N/A 20/12/2020– 20/12/2029 135.75 8,840 – – – – – 8,840 – 21/12/2020– 21/12/2023 21/12/2020 (1) N/A 21/12/2021– 21/12/2030 247.60 5,840 – 2,200 3,333 – – 3,640 – 24/12/2021– 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022– 24/12/2031 309.20 2,700 – – – – 2,700 2,700 2,700 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023– 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 1,600 – – – 1,600 1,600 1,600 Total 45,080 1,600 2,200 3,333 – 7,000 44,480 7,000 James M. Daly 28/06/2018– 28/06/2021 28/06/2018 (1) N/A 28/06/2019– 28/06/2028 80.82 – – – – – – – – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 209 |
| Information regarding the reported financial year Opening balance During the Year Closing balance Name of Board member Perfor-mance period Award date Vesting date End of retention period Exercise period Grant price in € Stock options held at the beginning of the year Stock options awarded Stock options exercised Stock options vested Stock options subject to a service condition Stock options awarded and unvested Stock options held at the end of the year Stock options subject to a retention period 21/12/2018– 21/12/2021 21/12/2018 (1) N/A 21/12/2019– 21/12/2028 86.32 – – – – – – – – 20/12/2019– 20/12/2022 20/12/2019 (1) N/A 20/12/2020– 20/12/2029 135.75 10,000 – 10,000 – – – – – 21/12/2020– 21/12/2023 21/12/2020 (1) N/A 21/12/2021– 21/12/2030 247.60 10,000 – – 3,333 – – 10,000 – 24/12/2021– 24/12/2024 24/12/2021 (2) 31/12/2024 24/12/2022– 24/12/2031 309.20 2,700 – – – – 2,700 2,700 2,700 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023– 23/12/2032 359.60 2,700 – – – – 2,700 2,700 2,700 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 1,600 – – – 1,600 1,600 1,600 Total 25,400 1,600 10,000 3,333 – 7,000 17,000 7,000 Camilla Sylvest 03/10/2022– 03/10/2025 03/10/2022 (2) 31/12/2025 03/10/2023– 03/10/2032 368.50 4,050 – – – – 4,050 4,050 4,050 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 1,200 – – – 1,200 1,200 1,200 Total 4,050 1,200 – – – 5,250 5,250 5,250 Ana Cespedes 23/12/2022– 23/12/2025 23/12/2022 (2) 31/12/2025 23/12/2023– 23/12/2032 359.60 4,050 – – – – 4,050 4,050 4,050 03/07/2023– 03/07/2026 03/07/2023 (2) 31/12/2026 03/07/2024– 03/07/2033 355.40 – 800 – – – 800 800 800 Total 4,050 800 – – – 4,850 4,850 4,850 1) 1/3rd of the stock options vests on the first anniversary of the date of grant and the remaining 2/3rd vests in equal monthly instalments (24 in total) over the next two years, each time upon the 1st day of each next month. 2) Stock options vest upon third anniversary of the grant. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Non-Executive Remuneration | 210 |
| 下表顯示了(I)在2023年1月1日持有的RSU,(Ii)在截至2023年12月31日的年度內授予非執行董事的RSU,以及(Iii)計劃在截至2024年12月31日、2025年12月31日、2026年12月31日和12月31日的年度歸屬的RSU,2027(以the year RSUs awarded RSUs vested RSUs subject數量表示): 有關報告的財政年度的信息 年度期初餘額 年度期末餘額 董事會成員業績獎勵日期名稱 授予 日期 保留期結束 保留時間為 開始 至 服務條件 的 Br}授予 和 未授予的 個RSU 在 年 結束時持有 個RSU 受 a 保留期 Peter K.M.Verhaeghe 24/12/2021-24/12/2025 24/12/2021(1)N/A 450-150-300- 23/12/2022-23/12/2026 23/12/2022(1)N/A-150-450 450- 03/07/2023-03/07/2023(1)N/A-350--350- 總計1,050 350 300-1,100 1,100- 沃納·蘭塔勒24/12/2021-28/02/2023 24/12/2021(1)N/A 450- 總計450-450- Steve Krognes 03/04/2023-03/2027 03/04/2023(1)N/A-525--525 525- Total-525--525 525- Pamela Klein 24/12/2021-24/12/2025 24/12/2021(1)N/A 450-150-300- 23/12/2022-23/12/2026 23/12/2022(1)N/A 600-150-450- 03/07/2023-03/07/2023(1)N/A-350-350- 總計1,050 350 300-1,100 1,100- J.Donald DeBethizy 24/12/2021-24/12/2025 24/12/2021(1)N/A 450-150-300- 23/12/2022-23/12/2026 23/12/2022(1)N/A 600-150-450- 03/07/2023-03/07/2027 03/07-350-350- 合計1,050 350 300-1,100 1,100– ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 argenx 2023年年度報告非執行薪酬|211 |
| 有關報告的財政年度的信息 年度期初餘額 年度期末餘額 董事會成員名稱績效期間獎勵日期 授予日期 結束 保留期 在the year RSUs awarded RSUs vested RSUs subject的 開始 持有的 service condition RSUs awarded and至 a RSU 未授權的 RSU在 年 結束時持有 RSU 受 a 保留期 Anthony A.Rosenberg 24/12/2021-24/12/2025 24/12/2021(1)N/A 450-150-300- 23/12/2022-23/12/2026/12/2022(1)N/A 600-150-450- 03/07/2023-03/07-2027 03/07/2023(1)N/A-350--350- 總計1,050 350 150-1,100 1,100- James M.Daly 24/12/2021-24/12/2021(1)N/A 450-150-300- 23/12/2022-23/12/2026 23/12/2022(1)N/A 600-150-450- 03/07/2023-03/07/2027 03/07/2023(1)N/A-350-350- 總計1,050 350 300-1,100 1,100- Camilla Sylvest 03/10/2022-03/10/2026 03/10/2022(1)N/A 900-225-675 675- 03/07/2023-03/07/2027(1)N/A-263-263-263- 總計900 263 225-938 938- Ana Cespedes 23/12/2022-23/122026年12月23日-2022年(1)N/A 900-225-675 675- 03/07/2023-03/2027 03/07/2023(1)N/A-175--175- 總計900 175 225-850 850- 1)RSU在四年內歸屬,在grant. ar Gr g oup enx Factors{br日期的每個週年日授予總額的四分之一}風險 去 公司 Verance Capital 分享財務 審核報表 財務非財務 信息 argenx 2023年年度報告非執行薪酬|212 |
| 持股要求 2023年,公司對非執行董事實行以下持股要求:每年3倍的現金預留。 最低股權要求在最長五年內積累,並在任職期間和之後的兩年內繼續申請。 2023財年,不向非執行董事發放遣散費。 2023年離職時的非執行股權待遇 ,公司更新了適用於非執行董事的股權激勵計劃的條款。 關於離職規則。特別是,根據股東對非執行董事股權實行多年服務式歸屬要求的潛在負面影響的反饋 ,股權激勵計劃進行了更新,以反映如果非執行董事被股東大會解職,他們將失去其未歸屬股權,但如果他們主動辭職或在任期結束時沒有申請重新任命 ,則不會失去他們的未歸屬股權。在建議的2024年薪酬政策草案中,本公司正進一步發展這項政策 並建議1年的歸屬年期與3年的歸屬後持有股權規定相結合。 應用與前董事會於完整服務期限後離職相同的原則,對Lanthaler先生而言, 本公司已同意,在其8年服務期限內授予的股權(2022年並無股權授予)被視為歸屬,但該等股權不得行使,除非於於授予時設定的歸屬條款完成後方可行使。為了具體解決既有但不可行使的股權的潛在税收成本,蘭塔勒先生被授予行使或出售其既有股權的這些部分的權利,以使他能夠支付即時税款 equity. ar Gr g oup enx Factors Risk Go Corporate vernance資本歸屬產生的負債 股票財務 審查報表 財務非財務信息 Argenx 2023年年度報告非執行薪酬|213 |
| 薪酬比率 整體薪酬比率 截至2023年12月31日的年度,支付給首席執行官(董事董事會中唯一的高管)的非股權薪酬的總支出為 1,285,056美元。下表顯示了CEO薪酬 過去五年的變化、公司股價的表現以及CEO的全職薪酬中值 (對於在 年內加入或離開我們的員工,按年率計算),CEO: 2019 2020 2021 2022 2023 CEO的基本工資(歐元)525,000 525,000 551,250 606,368 606,368美元CEO的基本工資(美元)$526,825 553,167 580,825 638,901 655,787 CEO的非股權薪酬(美元)(基本工資,短期現金 ,激勵支付給 員工的非股權工資中位數(美元)$121,603 163,062 157,349 153,193 159,500員工/首席執行官比率12%14%12%11%12% 支付給非執行董事的平均薪酬(美元)$60,372 57,925 54,484 48,587 59,230員工人數 12月31歐元336 650 843 1,148歐元泛歐交易所股票價格143.60 242.00 348.30 343.50(歐元)2012年12月31日泛歐交易所 (美元)2022年至2023年首席執行官與其他員工薪酬比率的提高是由於首席執行官基本工資保持不變時員工工資的增加。 以上非股權薪酬的比較是指支付給首席執行官的薪酬 ,公司唯一高管董事,以及支付給 名員工的薪酬中值。本公司選擇比較非股權薪酬,因為授予的股票期權數量與授予的薪酬方案的整體規模掛鈎 ,而股權成分的價值取決於公司股價、波動性和無風險比率的演變,而無風險比率在授予時是未知的,因此股票期權的前瞻性估值方法通常不能準確地 表示授予的實際經濟價值。在公允估值中使用的假設中,比利時受益人和非比利時受益人之間存在差異。有關評估這些獎項時使用的假設的説明,請參閲我們的合併財務報表第6節“合併財務報表”中的附註13。 3.4.6 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告薪酬比率|214 |
| 地區薪酬比率 由於員工分佈在多個大洲,因此 還應將上述比較分別與美國員工、歐盟員工和日本員工 進行比較。由於與歐盟相比,美國和日本業務部門的整體薪酬水平較高,因此,如果將CEO的薪酬與所有員工(大多數為歐盟公民)的薪酬中值進行比較(如上所述),或與美國和日本的員工進行比較,則薪酬比率存在顯著差異。以下信息僅供參考: 截至2023年12月31日的財年,員工非股權薪酬中位數與首席執行官的比率 所有員工13% 歐盟員工9% 美國員工21% 日本員工8% 2023財年支付的總僱傭成本(不包括任何與股票期權和RSU相關的成本)按地區分配如下: 截至12月31日的財政年度的總僱傭成本,2023年(百萬美元) 歐洲159.2北美130.2日本12.9以股票為基礎的支付比率 2019年2021年2022年2023年授予首席執行官的股票期權授予首席執行官80,000 50,000 25,000 25,000 30,000名員工的股票期權中值2,800 2,900 981 900 600員工/首席執行官4%6%4%4%2% 授予非執行董事的平均股票期權數量 授予非執行董事的股票期權中值10,000 10,000 2,869 3,086 1,550授予 員工2,800 2,900 981 900 600比率非執行董事/ }員工28%29%34%29%Argenx資本 股票財務 審核報表 財務非財務 信息 39% ar Gr g oup enx Factors Risk Go Corporate vernance 2023年年度報告薪酬比率|215 |
| Other Disclosures Remuneration by subsidiaries In fiscal year 2023, no remuneration was granted and allocated by subsidiaries or other companies whose financials are consolidated, other than the regular remuneration payments made by the entities with whom members of senior management have their employment contracts. No loans or guarantees In fiscal year 2023, no loans were granted to members of senior management and non-executive directors and no guarantees or the like have been granted in favor of any member of senior management or Board of Directors. Deviations In fiscal year 2023, the Company did not deviate from the decision-making process for the implementation of the 2021 Remuneration Policy for members of senior management and non-executive directors and no temporary deviations took place from the 2021 Remuneration Policy. Key terms of equity plan applicable to grants in 2023 Stock options granted pursuant to the Equity Incentive Plan shall vest with respect to one third of the shares upon the first anniversary of the date of grant, with the remaining two thirds vesting in 24 equal monthly instalments with the stock options fully vesting upon the third anniversary of the date of grant, subject, in each case, to the optionee’s continued status as a service provider. Stock options are exercisable when vested, and in any case not after the stock option expiration date included in each individual stock option grant, which is 10 years or in the case of Belgian tax resident employees, at their election either five years or 10 years from the date of grant. Each stock option shall be granted with an exercise price equal to the fair market value upon the date of grant and shall have a term equal to five or 10 years from the date of grant. Optionees may prefer to elect the five-year period as this may limit their personal tax obligations in respect of the stock option in respect to the jurisdiction where stock options are taxed at grant, compared to a ten-year stock option. Stock options granted to Belgian tax resident beneficiaries (including the CEO) are not exercisable prior to the fourth year following the year of the grant. Stock options granted to non-executive directors vest at once on the third anniversary of the date of grant. RSUs granted under the Equity Incentive Plan shall vest over a period of four years with respect to one fourth of the shares upon each anniversary of the date of grant. At the time of vesting, the holder of such RSUs receives shares in the share capital of the Company for free equal to the number equal of RSUs vested minus a certain number of shares required to cover employee taxes payable by us on behalf of the holder of RSUs, if applicable. Unvested equity incentives shall vest in the event of a (i) sale, merger, consolidation, tender offer or similar acquisition of shares or other transaction or series of related transactions as a result of which a change in control occurs, (ii) sale or other disposition of all or substantially all of the Company’s assets or (iii) the Company’s dissolution and/or liquidation. 3.4.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Disclosures | 216 |
| The Board of Directors, upon approval of a majority of the non-executive directors, may amend or terminate the Equity Incentive Plan or may amend the terms of the Equity Incentive Plan, or any outstanding stock options or RSUs, provided that the Company will compensate any affected individual for any direct negative impact of such amendment. Corporate Governance – Nasdaq Listing Rules As a foreign private issuer, the Nasdaq Listing Rules include certain accommodations in the corporate governance requirements that allow foreign private issuers to follow “home country” corporate governance practices in lieu of the otherwise applicable Nasdaq corporate governance standards. We intend to rely on certain exemptions for foreign private issuers and to follow Dutch corporate governance practices in lieu of the Nasdaq corporate governance rules. The following is a summary of the significant ways in which our corporate governance practices differ from those required by the Nasdaq Listing Rules with which we are not required to comply: · Quorum at Shareholder Meetings. In accordance with Dutch law and generally accepted business practices in the Netherlands, our Articles of Association do not provide quorum requirements generally applicable to general meetings of shareholders. To that extent, our practice varies from the requirement of Nasdaq Listing Rule 5620(c), which requires an issuer to provide in its bylaws for a generally applicable quorum, and that such quorum may not be less than one-third of the outstanding voting stock. · Solicitation of Proxies. Although we must provide shareholders with an agenda and other relevant documents ahead of any General Meeting, Dutch law does not have a regulatory regime for the solicitation of proxies, and the solicitation of proxies is not a generally accepted business practice in the Netherlands. Thus, our practice varies from the requirement of Nasdaq Listing Rule 5620(b). · Shareholder Approval. We follow certain Dutch shareholder approval requirements for the issuance of securities in connection with certain events such as the acquisition of stock or assets of another company, the establishment of or amendments to equity-based compensation plans for employees, a change of control of us and certain private placements. To this extent, our practice varies from the requirements of Nasdaq Rule 5635, which generally requires an issuer to obtain shareholder approval for the issuance of securities in connection with such events. · Distribution of Annual Reports. We do not follow Nasdaq Listing Rule 5250(d), which requires companies to make available copies of their annual reports containing audited financial statements to their shareholders. The distribution of our annual reports to shareholders is not required under Dutch corporate law or Dutch securities laws. Furthermore, it is generally accepted business practice for Dutch companies not to distribute annual reports. In part, this is because the Dutch system of bearer shares has made it impractical to keep a current list of holders of the bearer shares in order to distribute the annual reports. Instead, we make our Annual Report available at our corporate head office in the Netherlands (and at the offices of our Dutch listing agent as stated in the convening notice for the meeting) no later than 42 days prior to convocation of any annual General Meeting. In addition, we post a copy of our annual reports on our website prior to our annual General Meeting. 3.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Corporate Governance | 217 |
| 股份所有權 有關我們的董事和執行委員會成員的股份所有權的信息,請參閲“薪酬報告和薪酬報表” 部分和“股份類別和主要股東”小節“大股東”。 內幕交易 我們制定了符合MAR的內幕交易政策。內幕交易政策 旨在對內幕信息保密(根據MAR的定義),避免 操縱市場,並遵守Argenx在MAR、 交易法和其他適用證券法律下的義務。 網絡安全 信息安全風險管理和 戰略 我們的風險管理方法旨在識別、評估、區分優先順序和管理可能影響我們執行公司戰略和實現業務目標的能力的重大風險敞口。作為我們的信息安全和隱私計劃信息安全和管理系統(ISMS)的一部分,我們執行風險評估,在風險評估中,我們映射 並確定通過下述流程確定的信息安全風險的優先順序, 包括與我們使用第三方服務提供商相關的風險。這些評估 為我們的ISMS戰略和監督流程提供信息,並作為我們更廣泛的企業風險管理的一部分與其他企業風險一起 。我們將信息安全風險視為我們面臨的關鍵風險類別之一。IT系統供應商需要接受安全審查和 審核。有關我們面臨的網絡安全相關風險的更多信息,請參閲 第2.7.4節“如果系統出現故障,或者 未經授權或不當使用或訪問我們的系統,我們的業務和運營可能會受到影響”。 我們評估、識別和管理信息安全風險和 漏洞的流程作為ISMS的一部分嵌入到我們的業務中。除其他事項外,我們 對我們的信息系統進行審核和測試(包括由 獨立第三方顧問進行審查和評估,他們評估和報告我們的安全措施的成熟度,並幫助確定需要繼續關注和改進的領域),並審查我們參與的政府實體和其他 組織發佈的信息安全威脅信息。我們為我們的 員工進行數據安全問題培訓,以提高對潛在數據安全風險的認識和警覺,並將數據隱私納入我們的整體合規培訓中,例如通過針對 員工和承包商的隱私特定培訓。還定期實施釣魚培訓,其中包括 模擬釣魚電子郵件,以測試員工的警覺性。此外,員工需要閲讀 並確認與其特定角色相關的信息安全策略。我們還實施並維護了信息安全事件響應計劃,其中包括 對信息安全事件進行分類、評估嚴重性、上報、遏制、調查和補救的流程。以及遵守可能適用的法律義務 並減輕對品牌和聲譽的損害。 3.6 3.7 3.8 3.8.1 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告股權|218 |
| Information Security Governance and Oversight Our ISMS enables our Board of Directors to establish a mutual understanding with our senior management team of the effectiveness of our information security risk management practices and capabilities, including the division of responsibilities for reviewing our information security risk exposure and risk tolerance, tracking emerging information risks and ensuring proper escalation of certain key risks for periodic review by the Board of Directors and its committees. As part of its broader risk oversight activities, the Board of Directors oversees risks from information security threats, both directly and through the audit and compliance committee of the Board of Directors. The audit and compliance committee also oversees our internal control over financial reporting. As an element of its cybersecurity oversight activities, the audit and compliance committee regularly reviews the results of our enterprise risk assessments, including information security risk assessments, as well as management's strategies to detect, monitor and manage such risks and related risk assessment and risk management policies. Our ISMS contains provisions regarding reporting to the Global Risk Management Committee. Additionally, the data protection officer (the DPO) provides regular updates to senior management, and the audit and compliance committee as a component of the audit and compliance committee’s compliance updates. The DPO also regularly reports to the Global Corporate Compliance Committee, the Global Risk Management Committee and the General Counsel on matters such as the status of the organizational privacy plan, data breaches and routine programs. In addition to these regularly scheduled updates from the DPO, the Global Head of Business Information Systems reports to the audit and compliance committee or the full Board of Directors, as appropriate, on how certain information security risks are being managed and progress towards agreed mitigation goals, as well as any potential material risks from cybersecurity threats that have been detected by the information security team. Our information security team is responsible for day-to-day identification, assessment and management of the information security risks we face. Our Global Head of Business Information Systems has 32 years of experience in information management systems and the managers reporting to the Global Head of Business Information Systems have over 40 cumulative years of experience in information security. Our incident response and data breach procedures are designed for the timely detection, reporting, and investigation of all security incidents, as well as the timely notification of any reportable breaches (including any material cybersecurity incidents and personal data breaches) to the competent authorities and the timely communication to the affected individuals, where relevant. We maintain records of breaches on our quarterly corporate risk dashboard and our personal data breach register, and we monitor and regularly report our security and data breach metrics to senior management, including the audit and compliance committee of our Board of Directors, the Global Corporate Compliance Committee, and the Global Risk Management Committee. In addition to the ordinary-course Board of Directors and audit and compliance committee reporting and oversight described above, we also maintain disclosure controls and procedures designed for prompt reporting to the Board of Directors and timely public disclosure, as appropriate, of material events covered by our risk management framework, including information security risks. 3.8.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Cybersecurity | 219 |
| Risk Appetite & Control Before reading this section, please carefully review the following cautionary statement: In this section we will make the required disclosures regarding our risk appetite and mitigating actions. We fully take the risk mitigation actions and risk management described in this section into account while preparing the description of the main risks and uncertainties we face, as set out in section “Risk Factors”. Any mitigating language used in this section does not have any impact on the risks and uncertainties we face or their potential adverse effects as they are described in section “Risk Factors”. Section “Risk Factors” describes the main risks and uncertainties we face already fully having taken into account our risk management and the risk mitigating actions described herein. Introduction This section provides a general description of our willingness to mitigate the risks and uncertainties we face (also called our ‘risk appetite’), and to give a description of the mitigating actions we have taken with regard to our most relevant risks. General Description of Our Risk Appetite Our risk appetite serves as a guideline to determine the measures we may take in mitigating some of the risks and uncertainties we face. Our risk appetite is aligned with our strategy and priorities. The business we operate in is inherently high-risk. In general, we are willing, and in our view required, to take significant risks to be able to operate successfully in our line of business. Some of the risks and uncertainties we face are entirely outside of our control whereas others may be influenced or mitigated. The process of developing, implementing and improving risk management procedures remains an ongoing effort. In accordance with guideline 400.110c of the Dutch Counsel for Annual Reporting (Raad voor de Jaarverslaggeving), this risk management section provides an overview of the risk mitigating actions taken or planned to be taken by us. The mentioning of these mitigating actions may not in any way be viewed as an implied or express guarantee that such mitigation will in practice be effective in limiting the risk exposure and/or the potential damage to us from any such risk materializing. 3.9 3.9.1 3.9.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Risk Appetite & Control | 220 |
| Controlling Actions We Take with Regard to Our Most Relevant Risks and Uncertainties The following is a description of the main risks and uncertainties we face (being the first risk of each category of risk factors set out in section “Risk Factors”) and a description of the measures we took to control them. A description of the expected impact upon materialization of these risks is included for each risk in section “Risk Factors”. RISK FACTOR MEASURES TAKEN TO CONTROL THESE RISKS We have incurred significant losses since our inception and expect to incur losses for the foreseeable future. We may never achieve or sustain profitability. We have adopted a business model and strategic portfolio management approach to spread risks over wholly-owned programs as well as partnered programs, and to manage risks within our own proprietary product candidates pipeline. We continue to conduct research and development, preclinical testing, clinical trials and regulatory compliance activities as well as the continued commercialization of VYVGART and other products candidates, for current and future indications, and we intend to continue our efforts to expand our sales, marketing and distribution infrastructure. We will face significant challenges in successfully commercializing our products and additional product candidates after they are launched. We plan to focus on the successful development and commercialization of the products and product candidates after they are launched. We aim to expand our sales and marketing organization, enter into collaboration arrangements with third parties, outsource certain functions to third parties, or use some combination of each. We have already built, and continue to expand, our sales forces in certain of the VYVGART Approved Countries and plan to further develop our sales and marketing capabilities to promote our products, and product candidates, including new indications, if and when marketing approval has been obtained in other relevant jurisdictions. We are subject to healthcare laws, regulation and enforcement. The failure to comply with these laws could harm our results, operations and/or financial conditions. We are continuing to build and refine an internal program to ensure compliance with the different healthcare, compliance and reporting laws and regulations in multiple jurisdictions. 3.9.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Risk Appetite & Control | 221 |
| Failure to successfully identify, select and develop VYVGART in other indications, or additional products or product candidates could impair our ability to grow. We remain committed to using technology and contracting with parties that are able to achieve the level of sophistication we need to accurately and reliably identify, select and develop efgartigimod in other indications, additional products or product candidates. We expect our spending to continue to increase as we expand our global commercial infrastructure and drug inventory for VYVGART for the treatment of gMG, the progress of our clinical-stage pipeline, including ongoing clinical trials for five indications of efgartigimod. We rely, and expect to continue to rely, on third parties to conduct some of our research activities and clinical trials and for parts of the development and commercialization of our existing and future research programs, products and product candidates. If our relationships with such third parties are not successful, our business may be adversely affected. We endeavor to meet our contractual obligations and any relevant milestone achievements under our collaboration contracts, maintain a rich pipeline of possible collaboration partners as well as foster good relationships with existing and potential future collaboration partners in order to limit reliance on a limited number of collaboration partners. Furthermore, third-party contractor selection and management is subject to our quality management system. Customary contractual agreements are put in place in an effort to protect us from under-performance. We are typically spreading operational risks over various service providers. Project management belongs to our core internal competences. Our employees may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements, or insider trading violations, which could significantly harm our business. We have adopted a Code of Conduct, that is applicable to all of our employees and directors, which addresses the key risks related to potential breaches of ethical standards. All employees have accepted and are trained (and retrained annually) on our Code of Conduct. We expect all newcomers to accept, and commit to, the contents of the Code of Conduct. To increase compliance and ensure our colleagues know where to go with questions on the Code of Conduct and its application, we have established the argenx COMPASS Helpline, where our employees can raise any concerns they may have regarding potential violations of our policy confidentially or anonymously (to the extent allowed by law). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Risk Appetite & Control | 222 |
| Failure to adequately enforce or protect our intellectual property rights in products, product candidates and platform technologies could adversely affect our ability to maximize the value for patients in our marketed products and product candidates. We strive to protect the proprietary technologies that we believe are important to our business, including pursuing and maintaining patent protection intended to cover the platform technologies incorporated into, or used to produce, our product candidates, the compositions of matter of our product candidates and their methods of use, as well as other inventions that are important to our business. In addition to patent protection, we also rely on trademarks and trade secrets to protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection, including certain aspects of our llama immunization and antibody affinity maturation approaches. Our future growth and ability to compete depends on retaining our key personnel and recruiting additional qualified personnel. We offer competitive remuneration packages and share-based incentives in the form of the Equity Incentive Plan. We perform periodic benchmark analyses with an external service provider to ensure the competitiveness of the compensation offered to our key personnel in comparison to other (reference group) companies. We pay close attention to creating an environment that supports the further development of the talents of our key people. Material Impact of Risk Materialization in 2023 During the period between January 1, 2023 and December 31, 2023, we did not identify any material impact as a result of materialization of previously identified risks and uncertainties. Financial Risks and Controls In running our business, we seek to implement a sustainable policy regarding internal control and risk management. Our Board of Directors has delegated an active role to our audit and compliance committee in the design, implementation and monitoring of an internal risk management and control system to manage the significant risks to which we are exposed. Our financial reporting is structured within a tight framework of budgeting, reporting and forecasting. A distinction is made between reports for internal and external use. External reporting at group level consists of an annual report (in the form of this Annual Report), including financial statements audited by the independent auditor, as well semi-annual reporting and quarterly updates, containing summarized financial information. The external reports are based on the internal financial reporting. 3.9.4 3.9.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Risk Appetite & Control | 223 |
| 內部財務報告由廣泛的綜合月報組成,其中 將當前的發展與月度(累計)預算和以前的預測進行比較。此外,我們每個季度都會重申或更新對年度業績的預測,包括財年結束時的現金流狀況。季度預算是集團年度預算的一部分,該預算由我們的高級管理層每年編制,並由我們的董事會批准。我們專門的財務和行政部門 主要負責評估內部和外部報告草案,然後由董事會最終批准。 我們的董事會在所有正式董事會會議上討論集團的財務結果,這些會議是紀要的。 我們對財務報告的內部控制是內部控制的一個子集,包括 政策和程序,這些政策和程序: 與維護合理詳細、準確和公平地反映我們資產的交易和處置的記錄有關; ·提供合理保證,確保交易記錄為必要,以便根據國際會計準則理事會發布的和歐盟採納的《國際財務報告準則》編制我們的財務報表,並確保收入和支出僅由授權人員進行;以及 ·為防止或及時發現可能對財務報表產生重大影響的未經授權的資產收購、使用或處置提供合理保證。 由於我們擁有在美國證券交易委員會註冊的證券,並且是交易法12b-2規則 所指的大型加速申報機構,因此我們需要評估我們對財務報告的內部 控制的有效性,並就評估結果提供報告。我們的 董事會根據特雷德韋委員會的贊助組織委員會發布的《內部控制-綜合框架(2013)》中建立的標準,審查了對財務報告的內部控制,並聘請了外部顧問來幫助評估其控制的有效性。 我們的風險管理系統最近或當前的發展 我們關注主動風險管理,繼續將對我們的核心風險和不確定性的評估作為我們董事會的常設討論議題。此外,在2023年,我們在董事會的議程中增加了特定風險的季度更新,包括網絡安全、隱私和醫療保健合規風險。 3.9.6 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 argenx 2023年年度報告風險偏好與控制|224 |
| 一般 公司及其 股本 4.1公司法律資料226 4.2股本227 4.3股份類別及主要股東232 4.4持有證券權利的限制236 4.5股東大會,投票權和入場236 4.6反收購條款239 4.7外匯管制239 4.8公司章程修訂239 4.9透明度指令239 4.10荷蘭財務報告監督法240 4.11股息及其他分派240 4.12在清算時獲得盈餘的權利241 4.13擔保權的重大修改 持有人及所得款項用途242 4.14民事責任的執行242 4.15控制及程序244 4.16財務日曆2024 245 4 argenx 2023年年報股份資本|225 |
| 4 General Description of the Company and its Share Capital Legal Information on the Company General We were incorporated on April 25, 2008 in the Netherlands and under Dutch law. Our commercial name is ‘argenx’ and since April 26, 2017, our corporate name is ‘argenx SE’. We are a European public company (Societas Europaea or SE), with our corporate seat in Rotterdam, the Netherlands, are registered with the trade register of the Dutch Chamber of Commerce under number 24435214. Our registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands and our telephone number is +31 (0) 10 70 38 441. Our website address is http://www.argenx.com. Our LEI is 7245009C5FZE6G9ODQ71. Our ordinary shares are listed on Euronext Brussels under ISIN NL0010832176 under the symbol “ARGX” since July 10, 2014. The ADSs are listed on Nasdaq, under the symbol “ARGX” since May 18, 2017. Statutory/Corporate Objectives Pursuant to Article 3 of our Articles of Association, our corporate objectives are: (a) to exploit, including all activities relating to research, development, production, marketing and commercial exploitation; biological, chemical or other products, processes and technologies in the life sciences sector in general, and more specifically in the diagnostic, pharmaceutical, medical, cosmetic, chemical and agricultural sector; (b) to design and develop instruments which may be used in medical diagnosis and affiliated areas; (c) the worldwide distribution of, sale of and rendering services relating to our products and subsidiaries directly to customers as well as through third parties; (d) to incorporate, to participate in any way whatsoever, to manage, to supervise, to operate and to promote enterprises, businesses and companies; (e) to render advice and services to businesses and companies with which we form a group and to third parties; (f) to finance businesses and companies; (g) to borrow, to lend and to raise funds, including the issue of bonds, promissory notes or other securities or evidence of indebtedness as well as to enter into agreements in connection with the aforementioned; (h) to render guarantees, to bind us and to pledge our assets for obligations of the companies and enterprises with which we form a group and on behalf of third parties; (i) to obtain, alienate, manage and exploit registered property and items of property in general; (j) to trade in currencies, securities and items of property in general; (k) to develop and trade in patents, trademarks, licenses, know-how and other industrial property rights; and (l) to perform any and all activities of industrial, financial or commercial nature, as well as everything pertaining the foregoing, relating thereto or conductive thereto, all in the widest sense of the word. 4.1 4.1.1 4.1.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Legal Information on the Company | 226 |
| Share Capital Authorized and Issued Share Capital Under Dutch Law, a company’s authorized share capital sets out the maximum amount and number of shares that it may issue without amending its articles of association. Our Articles of Association provide for an authorized share capital in the amount of €9.0 million divided into 90 million shares, each with a nominal value of €0.10. All issued and outstanding shares have been fully paid up and the shares are held in dematerialized form. As of December 31, 2023 our issued and paid up share capital amounted to €5,919,488 ($7,058,118), represented by 59,194,488 ordinary shares with a nominal value of €0.10, each representing an identical fraction of our share capital. As of December 31, 2023, neither we nor any of our subsidiaries held any of our own shares. Stock Options and Restricted Stock Units In addition to the shares already outstanding, we have granted stock options which upon exercise will lead to an increase in the number of our outstanding shares. 61,056 stock options were granted on April 3, 2023, 629,121 on July 3, 2023, 74,529 on October 2, 2023 and 79,305 on December 31, 2023. A total of 5,118,949 stock options (where each stock option entitles the holder to subscribe for one new ordinary share) were outstanding and granted as of December 31, 2023. Upon exercise of these 5,118,949 stock options, we will receive a total amount of €1,184 million ($1,308 million) in stock option exercise price, thereby increasing our share capital and share premium by the same amount. Further, we have granted RSUs which upon vesting will lead to an increase in the number of our outstanding shares. 13,719 RSUs were granted on April 3, 2023, 143,402 on July 3, 2023, 17,306 on October 2, 2023 and 17,810 on December 22, 2023. A total of 442,322 RSUs (where the holder receives an equal number of new ordinary shares, minus a certain number of shares required to cover certain costs, if applicable) were outstanding and granted as of December 31, 2023. Apart from the stock options and RSUs granted under our Equity Incentive Plan, we do not currently have other stock options, RSUs, options to purchase securities, convertible securities or other rights to subscribe for or purchase securities outstanding. For stock option information through December 31, 2023, see Note 13 “Share-based payments” in our consolidated financial statements in section “Consolidated Financial Statements – for the year ended December 31, 2023”. American Depositary Shares In connection with our initial public offering on Nasdaq, the Bank of New York Mellon, as depositary, registered and delivered ADSs. Each ADS represents one share (or a right to receive one share) deposited with ING Bank N.V., as custodian for the depositary in the Netherlands. Each ADS also represents any other securities, cash or other property which may be held by the depositary. The deposited shares together with our other securities, cash and other property held by the depositary, are referred to as the deposited securities. The depositary’s office at which the ADSs are administered is located at 101 Barclay Street, New York, New York 10286. The Bank of New York Mellon’s principal executive office is located at 225 Liberty Street, New York, New York 10286. 4.2 4.2.1 4.2.2 4.2.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Capital | 227 |
| A deposit agreement among us, the depositary, ADS holders and all other persons indirectly or beneficially holding ADSs sets out ADS holder rights as well as the rights and obligations of the depositary. New York law governs the deposit agreement and the ADSs. Fees and Charges Persons depositing or withdrawing shares or ADS holders must pay: For: $5.00 (or less) per 100 ADSs (or portion of 100 ADSs) Issuance of ADSs, including issuances resulting from a distribution of shares or rights or other property Cancellation of ADSs for the purpose of withdrawal, including if the deposit agreement terminates $.05 (or less) per ADS Any cash distribution to ADS holders A fee equivalent to the fee that would be payable if securities distributed to you had been shares and the shares had been deposited for issuance of ADSs Distribution of securities distributed to holders of deposited securities (including rights) that are distributed by the depositary to ADS holders $.05 (or less) per ADS per calendar year Depositary services Registration or transfer fees Transfer and registration of shares on our share register to or from the name of the depositary or its agent when you deposit or withdraw shares Expenses of the depositary Cable, telex and facsimile transmissions (when expressly provided in the deposit agreement) Converting foreign currency to USDs Taxes and other governmental charges the depositary or the custodian has to pay on any ADSs or shares underlying ADSs, such as stock transfer taxes, stamp duty or withholding taxes As necessary Any charges incurred by the depositary or its agents for servicing the deposited securities As necessary 4.2.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Capital | 228 |
| 託管機構直接向投資者收取交付和交出美國存託憑證的費用 存放股票或交出美國存託憑證的目的是為了提款或向其代理的中介機構收取費用 。託管人通過從分配的金額中扣除 這些費用或出售一部分可分配財產來支付費用來收取向投資者進行分配的費用。託管人可以通過從現金分配中扣除 ,或直接向投資者收費,或向代表他們的參與者的賬簿記賬系統收費,來收取託管服務的年費。託管銀行可通過從應付給美國存托股份持有人的任何現金分配(或出售一部分證券或其他可分配財產)中扣除 有義務支付這些費用的方式來收取任何費用。託管銀行 一般可以拒絕提供吸引費用的服務,直到支付這些服務的費用為止。 託管銀行可能會不時向我們付款,以償還我們因建立和維護美國存托股份計劃而產生的費用和支出,免除託管銀行向我們提供的服務的費用和開支,或分享從美國存托股份持有人那裏收取的費用。在履行存款協議項下的職責時,託管機構可以使用由託管機構擁有或與其有關聯的經紀商、交易商、外匯交易商或其他服務提供者,這些機構可以賺取或分享手續費、利差或佣金。 託管機構可以自己或通過其任何關聯機構兑換貨幣,在這種情況下,託管機構將作為其賬户的委託人,而不是作為代理人、顧問、經紀人或受託機構為 任何其他人賺取收入,包括但不限於交易利差,它將為自己的賬户保留這些收入。除其他外,收入的計算依據是根據存款協議進行的貨幣兑換的匯率與保管人或其附屬機構在為自己的賬户購買或出售外幣時收到的匯率之間的差額。託管銀行不表示根據存款協議在任何貨幣兑換中使用或獲得的匯率 將是當時可獲得的最優惠匯率,或確定該匯率的方法將對美國存托股份持有者最有利,但須遵守存款協議規定的 託管機構的義務。用於確定貨幣兑換中使用的匯率的方法可根據要求提供。 在2023年期間創建的新股由於股票期權的行使和我們股權激勵計劃下RSU的歸屬,在2023年創建了1,216,999股新股。 2023年7月24日,我們通過全球發售完成了2,581,633股普通股的發行。此次全球發售包括由美國和歐洲經濟區以外的某些其他國家的美國存託憑證代表的普通股發行,以及同時在歐洲經濟區和英國進行的普通股私募。因此,我們從此次發行中獲得了13億美元的毛收入,減少了6590萬美元的承銷商 折扣和佣金,以及發售費用,其中80萬美元已從股權中扣除。此次發行的現金淨收益總額為1.2億美元billion. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告股票資本|229 |
| The following table shows the developments in our share capital for the fiscal year ending December 31, 2023 and on February 20, 2024: Number of shares outstanding on December 31, 2021 51,668,315 Number of shares outstanding on December 31, 2022 55,395,856 Exercise of stock options in 2023 1,137,439 Vesting of RSUs 79,560 Global public offering in Euronext and Nasdaq on July 17, 2023 2,244,899 Over-allotment option exercised by underwriters on July 19, 2023 336,734 Number of shares outstanding on December 31, 2023 59,194,488 Exercise of stock options in January 2024 106,617 Exercise of stock options in February 2024 2,277 Number of shares outstanding on February 20, 2024 59,302,232 Issue of Shares The Articles of Association provide that shares may be issued or rights to subscribe for our shares may be granted pursuant to a resolution of the shareholders at a General Meeting, or alternatively, by our Board of Directors if so designated by the shareholders at a General Meeting. If the Board of Directors is designated by the shareholders at a General Meeting to issue shares or grant rights to subscribe for shares, the shareholders are not permitted to also do so as long as the designation of the Board of Directors is in effect. A resolution of the shareholders at a General Meeting to issue shares, to grant rights to subscribe for shares or to designate our Board of Directors as the corporate body authorized to do so can only take place at the proposal of our Board of Directors. Shares may be issued or rights to subscribe for shares may be granted by resolution of our Board of Directors, if and insofar as our Board of Directors is designated to do so by the shareholders at a General Meeting. Designation by resolution of the shareholders at a General Meeting cannot be withdrawn unless determined otherwise at the time of designation. The scope and duration of our Board of Directors’ authority to issue shares or grant rights to subscribe for shares (such as granting stock options or issuing convertible bonds) is determined by a resolution of the shareholders at the General Meeting and relates, at the most, to all unissued shares in our authorized capital at the relevant time. The duration of this authority may not exceed a period of five years. Designation of our Board of Directors as the body authorized to issue shares or grant rights to subscribe for shares may be extended by a resolution of the shareholders at a General Meeting for a period not exceeding five years in each case. The number of shares that may be issued is determined at the time of designation. A resolution of our Board of Directors to issue shares and to grant rights to subscribe for shares can only be taken with the consent of the majority of the non-executive directors. The 2023 General Meeting designated our Board of Directors as the corporate body competent to issue additional shares and grant rights to subscribe for shares up to a maximum of 10% of the outstanding capital at the date of the 2023 General Meeting, and to limit or exclude pre-emptive rights of shareholders for such shares with the prior consent of the majority of the non-executive directors for a period of 18 months. 4.2.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Capital | 230 |
| Pre-Emption Rights Dutch law and the Articles of Association give shareholders pre-emptive rights to subscribe on a pro rata basis for any issue of new shares or, upon a grant of rights, to subscribe for shares. Holders of shares have no pre-emptive rights upon (i) the issue of shares against a payment in kind (being a contribution other than in cash); (ii) the issue of shares to our employees or the employees of a member of our group; and (iii) the issue of shares to persons exercising a previously granted right to subscribe for shares. A shareholder may exercise pre-emptive rights during a period of at least two weeks from the date of the announcement of the issue of shares. Pursuant to the Articles of Association, the shareholders at a General Meeting may restrict or exclude the pre-emptive rights of shareholders. A resolution of the shareholders at a General Meeting to restrict or exclude the pre-emptive rights or to designate our Board of Directors as our corporate body authorized to do so, may only be adopted on the proposal of our Board of Directors with the consent of the majority of the non-executive directors. A resolution of the shareholders at a General Meeting to exclude or restrict pre-emptive rights, or to authorize our Board of Directors to exclude or restrict pre-emptive rights, requires a majority of at least two-thirds of the votes cast, if less than 50% of our issued and outstanding share capital is present or represented at the General Meeting. With respect to an issuance of shares pursuant to a resolution of our Board of Directors, the pre-emptive rights of shareholders may be restricted or excluded by resolution of our Board of Directors if and insofar as our Board of Directors is designated to do so by the shareholders at a General Meeting. A resolution of our Board of Directors to restrict or exclude pre-emptive rights can only be taken with the consent of the majority of the non-executive directors. The designation of our Board of Directors as the body competent to restrict or exclude the pre-emptive rights may be extended by a resolution of the shareholders at a General Meeting for a period not exceeding five years in each case. Designation by resolution of the shareholders at a General Meeting cannot be withdrawn unless determined otherwise at the time of designation. Please refer to section “Issue of Shares” with respect to the current right of the Board of Directors to limit or exclude pre-emptive rights. Acquisition of Shares in our Capital We may not subscribe for our own shares on issue. We may acquire fully paid-up shares at any time for no consideration or, if: · our shareholders’ equity less the payment required to make the acquisition, does not fall below the sum of called-up and paid-in share capital and any statutory reserves; · we and our subsidiaries would thereafter not hold shares or hold a pledge over shares with an aggregate nominal value exceeding 50% of our issued share capital; and · our Board of Directors has been authorized thereto by the shareholders at a General Meeting. As part of the authorization, the shareholders at a General Meeting must specify the number of shares that may be repurchased, the manner in which the shares may be acquired and the price range within which the shares may be acquired. An authorization by the shareholders at a General Meeting to our Board of Directors for the repurchase of shares can be granted for a maximum period of 18 months. No authorization of the shareholders at a General Meeting is 4.2.6 4.2.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Capital | 231 |
| 如果我們收購普通股,意在根據股權激勵計劃將該等普通股轉讓給我們的員工,則需要。只有在非執行董事的多數同意下,我們的董事會才能作出回購股份的決議。我們以我們的自有股本持有的股份不具有任何分配權。 此外,我們或我們的子公司持有的任何股份不得行使投票權,除非該等股份受用益物權或以我們或我們的子公司以外的 人為受益人的質押的約束,並且投票權在我們或我們的子公司獲得該等股份之前已歸屬質權人或用益物權人。吾等或吾等子公司均不得對吾等或吾等子公司 有用益物權或質權的股份行使投票權。 減少股本 股東在股東大會上可根據吾等董事會的建議,通過註銷股份或修改 公司章程以降低股份面值的方式決議削減已發行股本。 股份類別及主要股東 股東 於2月20日,2024年我們的已發行股本為5,930,223.20歐元,由59,302,232股普通股代表。只有一類股票(普通股, 包括以美國存託憑證為代表的普通股),任何普通股都沒有附帶任何特別權利,也沒有我們的任何股東的特別股東權利,包括投票權。每位股東有一票投票權。 披露持股情況 根據DFSA,任何人直接或間接收購或處置公司資本、投票權或總空頭頭寸的(實際或視為)權益,必須 立即以標準格式向荷蘭金融市場管理局(Stichting Autoriteit Financiële Markten,AFM)發出書面通知,如果由於此類收購或處置,該人持有的資本權益或投票權的百分比達到、超過或低於以下門檻:5%、10%、15%、20%、25%、30%、 40%、50%、60%、75%和95%。 在計算資本權益或投票權的百分比時,必須考慮以下 權益:(I)任何人直接持有(或獲得或處置)的股份和/或投票權;(2)該人的受控實體或第三方為該人的賬户持有(或獲得或處置)的股份或投票權;(3)該人與其訂立口頭或書面投票協議的第三方持有(或獲得或處置)的投票權;(4)根據一項協議獲得的投票權,該協議規定以支付為代價臨時轉讓投票權;(V)上述人士或上述任何受控實體或第三方可根據任何認購權或其他收購股份權利獲得的股份;(Vi)決定某些現金結算金融工具價值的股份,如差價合約和總回報互換 掉期;(Vii)交易對手行使認沽期權時必須收購的股份;和 (Viii)作為另一份合同的標的的股票,其經濟狀況類似於直接或間接持有這些股票。 4.2.8 4.3 4.3.1 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 argenx 2023年年度報告股票類別和主要股東 |
| Controlled entities (gecontroleerde ondernemingen) within the meaning of the DFSA do not themselves have notification obligations under the DFSA as their direct and indirect interests are attributed to their (ultimate) parent. If a person who has a 3% or larger interest in the company’s share capital or voting rights ceases to be a controlled entity it must immediately notify the AFM and all notification obligations under the DFSA will become applicable to such former controlled entity. Special rules apply to the attribution of shares and/or voting rights which are part of the property of a partnership or other form of joint ownership. A holder of a pledge or right of usufruct in respect of shares can also be subject to notification obligations, if such person has, or can acquire, the right to vote on the shares. The acquisition of (conditional) voting rights by a pledgee or beneficial owner may also trigger notification obligations as if the pledgee or beneficial owner were the legal holder of the shares and/or voting rights. Furthermore, when calculating the percentage of capital interest a person is also considered to be in possession of shares if (i) such person holds a financial instrument the value of which is (in part) determined by the value of the shares or any distributions associated therewith and which does not entitle such person to acquire any shares, (ii) such person may be obliged to purchase shares on the basis of an option, or (iii) such person has concluded another contract whereby such person acquires an economic interest comparable to that of holding a share. Any person whose interest in the capital, voting rights or gross short position in the Company meets, exceeds or falls below one or several of the above-mentioned thresholds due to a change in the Company’s outstanding capital, or in voting rights attached to the shares as notified to the AFM by the Company, should notify the AFM no later than the fourth trading day after the AFM has published the notification by the Company. Furthermore, each director must notify the AFM of each change in the number of shares he or she holds and of each change in the number of votes he or she is entitled to cast in respect of our issued and outstanding share capital, immediately after the relevant change. The AFM does not issue separate public announcements of the notifications. It does, however, keep a public register of and publishes all notifications made pursuant to the DFSA at its website (www.afm.nl). Third parties can request to be notified automatically by email of changes to the public register in relation to a particular company’s shares or a particular notifying party. Non-compliance with these notification obligations is an economic offence and may lead to criminal prosecution. The AFM may impose administrative penalties for non-compliance, and the publication thereof. In addition, a civil court can impose measures against any person who fails to notify or incorrectly notifies the AFM of matters required to be notified. A claim requiring that such measures be imposed may be instituted by us, or by one or more of our shareholders who alone or together with others represent at least 3% of our issued and outstanding share capital of or voting rights. The measures that the civil court may impose include: · an order requiring the person with a duty to disclose to make the appropriate disclosure; · suspension of the right to exercise the voting rights by the person with a duty to disclose for a period of up to three years as determined by the court; · voiding a resolution adopted by the shareholders at a General Meeting, if the court determines that the resolution would not have been adopted but for the exercise of the voting rights of the person with a duty to disclose, or suspension of a resolution adopted by the shareholders at a General Meeting until the court makes a decision about such voiding; and ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Classes and Principal Shareholders | 233 |
| · an order to the person with a duty to disclose to refrain, during a period of up to five years as determined by the court, from acquiring shares or voting rights in the company. Shareholders are advised to consult with their own legal advisors to determine whether the notification obligations apply to them. Short positions Pursuant to EU Regulation No. 236/2012, each person holding a net short position attaining 0.2% of our issued share capital must report it to the AFM. Each subsequent increase of this position by 0.1% above 0.2% will also have to be reported. Each net short position equal to 0.5% of our issued share capital and any subsequent increase of that position by 0.1% will be made public via the AFM short selling register. To calculate whether a natural person or legal person has a net short position, their short positions and long positions must be set off. A short transaction in a share can only be contracted if a reasonable case can be made that the shares sold can actually be delivered, which requires confirmation of a third party that the shares have been located. The notification shall be made no later than 15:30 central European time on the following trading day. Furthermore, each person holding a gross short position in relation to our issued share capital that reaches, exceeds or falls below one of the following thresholds: 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, 50%, 60%, 75% and 95%, must immediately give written notice to the AFM. If a person’s gross short position reaches, exceeds or falls below one of the abovementioned thresholds as a result of a change in our issued share capital, such person is required to make a notification not later than on the fourth trading day after the AFM has published our notification in the public register of the AFM. The AFM keeps a public register of the short selling notifications. Shareholders are advised to consult with their own legal advisors to determine whether any of the above short selling notification obligations apply to them. The duty to notify applies to legal entities as well as natural persons. 4.3.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Classes and Principal Shareholders | 234 |
| 大股東 下表列出了有關我們 普通股實益所有權的信息,適用於已通知AFM其在公司的重大 權益佔我們已發行普通股總數的3%或更多的個人和實體,2024年. 實益所有人股份名稱 3%或更大股東* 股份數量 資本 權益 投票權數量 投票權 Artisan Investments GP LLC 2,674,1461)4.89%{674,1461)4.89% 百利吉福公司0 0.00%2,966,2162)6.24% 貝萊德,公司2,847,0063)5.09%3,387,4033)6.05% 資本研究和管理公司0 0.00%1,884,7064)3.19% FMR LLC 5,819,6615)9.93%5,814,7765)9.92% Janus Henderson Group plc,784,7236)3.02%1,784,7236)3.02% T.Rowe Price Group,公司6,167,2747)10.42%6,038,6867)10.20% 先鋒集團1,978,4648)4.16%08)0.00% 惠靈頓 管理集團有限責任公司0 0%1,777,5639)3.00% 1)包括46,766股普通股和2,627,380股,根據AFM備案文件,存託憑證(根據AFM備案文件,該實體可以在存託憑證上行使同等數量的投票權)。 2)包括2,966,216股普通股的投票權。 3)由2,172,838股普通股組成根據AFM備案文件,有2,651,688項投票權可由 該實體行使,673,904份,存託憑證(根據AFM備案文件,該實體可在其上行使735,451項投票權 )和264份差價合同(根據AFM備案文件,該實體可在其上行使同等數量的投票權)。 4)包括119,041股普通股和1,765,665股美國存託憑證的投票權。該實體可行使5,814,776投票權。 6)由10,882股普通股和1,773,841股美國存託憑證組成。 7)由10,100股普通股和6,157,174股美國存託憑證組成(根據AFM備案文件,該實體可行使6,028,586投票權)。 8)由1,978,464股普通股組成(根據AFM備案文件,該實體不能對其行使投票權)。 9)由1,520,216股普通股和257,347股美國存託憑證組成。 *根據所報告的證券數量,當時,向AFM提交了最新的透明度通知。實際利益可能會有所不同,因為重大權益的持有人只有在股本和/或投票權的百分比發生任何變化時才有義務通知AFM 如果該持有人直接或間接達到、超過或低於上述任何門檻。 截至2024年2月20日,未償還的股票期權和RSU總數為 4,999,378個股票期權和439,161個RSU。 截至本年度報告日期,我們不直接或間接由任何股東擁有或控制,無論是單獨還是一致行動。我們不知道有任何 安排可能在隨後的日期導致我們公司控制權的變更。 除了通過提交給美國證券交易委員會及其任何修正案的AFM備案文件或Schedule 13D或13G備案文件公開披露的情況外,除所有權百分比的變化外 4.3.3 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告股票類別和主要股東|235 |
| result of the shares issued in connection with our initial and follow-on U.S. public offerings, we are not aware of any significant change in the percentage ownership held by the major shareholders listed above. The number of record holders in the U.S. is not representative of the number of beneficial holders nor is it representative of where such beneficial holders are resident since many of these ordinary shares were held by brokers or other nominees. At February 20, 2024, assuming that all of our ordinary shares represented by ADSs are held by residents of the U.S., we estimate that approximately 53.61% of our outstanding ordinary shares were held in the U.S. by approximately one institutional holder of record, which is the Bank of New York Mellon as depositary of the ADSs. As of the date of this Annual Report, as far as we are aware, there are no direct or indirect relationships between us and any of our significant shareholders. Limitations on the right to hold securities Neither Dutch law nor our Articles of Association impose any general limitation on the right of non-residents or foreign persons to hold our securities or exercise voting rights on our securities other than those limitations that would generally apply to all shareholders. General Meeting, Voting Rights and Admission General Meetings are held at the place where the Company has its official seat, in Amsterdam or at Schiphol Airport (municipality of Haarlemmermeer), the Netherlands. The Articles of Association provide that at least one annual General Meeting shall be held within six months after the close of each fiscal year. Additional extraordinary General Meetings may be held whenever our Board of Directors deems such to be necessary. Shareholders representing alone or in aggregate at least one-tenth of our issued and outstanding share capital may, pursuant to the DCC, request that a General Meeting be convened. If our Board of Directors has not taken the steps necessary to ensure that a General Meeting will be held within the relevant statutory period after the request, the requesting persons may, at his/her/their request, be authorized by a court in preliminary relief proceedings to convene a General Meeting. The court shall disallow the application if it does not appear that the applicants have previously requested our Board of Directors to convene a General Meeting and our Board of Directors has not taken the necessary steps so that a General Meeting could be held within six weeks after the request. Within three months of it becoming apparent to our Board of Directors that our equity has decreased to an amount equal to or lower than one-half of the paid-in and called-up capital, a General Meeting would be held to discuss any requisite measures. We will give notice of any General Meeting by publication on our website and furthermore, to the extent required, in another manner in accordance with the applicable stock exchange regulations. The notice convening any General Meeting must include, among other items, an agenda indicating the place and date of the meeting, the items for discussion and voting, the proceedings for registration including the registration date, as well as any proposals for the agenda made by the Board of Directors or shareholders holding at least 3% of the issued 4.4 4.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Limitations on the right to hold securities | 236 |
| 股本。對於年度股東大會,議程應包括(除其他事項外)通過年度帳目、分配我們的利潤以及與我們董事會的組成有關的建議,包括填補我們董事會的任何空缺。 根據荷蘭法律,持有我們至少3%已發行和已發行股本的股東有權要求我們的董事會將項目列入任何 股東大會的議程。我們的董事會必須同意這些請求,條件是:(I)請求是以書面形式提出的,並且是出於動機,以及(Ii)我們的董事會主席至少在股東大會日期前60天收到了請求。 除已列入 議程的項目外,不得就其他項目通過任何決議。根據DCGC的規定,股東只有在徵詢我們董事會的意見後才能將項目列入議程 。如果一個或多個股東打算 要求將可能導致公司戰略改變的項目列入議程,我們的董事會可以援引最長180天的響應時間,直至股東大會召開之日。此外,根據DCC,我們的董事會可以 啟用最長250天的法定冷靜期(Weettelijke Bedenktijd)。對於 本公司來説,這意味着新規則將適用於以下情況: ·股東請求本公司董事會召開股東大會審議任命、停職或解聘一名或多名董事的提案,或 修改公司章程中與此相關的一項或多項規定的提案;或 ·在未與 投標人和公司就發行股票達成協議的情況下宣佈或進行公司資本中的股票公開發行;以及 ·只有在我們的董事會也認為有關情況嚴重違反本公司及其關聯企業的利益的情況下。 如果我們的董事會啟用了這樣的冷靜期,這將導致 股東大會任命、停職或罷免董事(以及修改 公司章程)的權力被暫停。 股東大會由董事長主持,如果董事長缺席,則由副董事長主持。如果主席和副董事長均缺席,出席會議的非執行董事應指定其中一人為主席。董事會成員可出席股東大會。在這些會議中,他們擁有 諮詢投票。會議主席可酌情決定接納其他 人蔘加會議。 公司的外聘審計師應出席討論年度帳目的股東大會。 我們的董事會必須至少在大會召開前一天(目前為42天)發出股東大會通知。 股東(以及其他有投票權或會議權利的人)可以出席股東大會,在股東大會上發表講話。只要他們有這樣的權利, 親自或委託代表按比例行使其所持股份的投票權。股東 可以行使這些權利,如果他們是登記日期的股票持有人,目前是股東大會日前的第28天,而且他們或他們的代理人已 書面通知我們的董事會,他們打算在上述meeting. ar Gr g oup enx Factors Risk Go Corporate vernance資本通知中指定的日期之前在 地址和日期之前參加股東大會。 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告股東大會、投票權和准入|237 |
| 所有股東,以及產生對我們股份的投票權的每個用益物權和質權人,有權親自出席股東大會,或由書面授權的代表代表出席股東大會並按比例行使投票權。 如果股東是我們股份的持有者,則他們可以在荷蘭法律規定的記錄日期 行使他們的權利,目前是股東大會日前28天, 及其或其代表已以書面或任何其他電子方式通知吾等有意出席該股東大會,而該意向最終可於本公司董事會為此目的而設定的日期 以書面形式轉載,該日期不得早於該股東大會前第七天,並註明該人士的姓名及股份數目 該人士可於該股東大會上行使投票權及/或會議權利 。召集通知應載明登記日期及有權出席股東大會的人士登記及行使權利的方式。 每股普通股賦予持有人在股東大會上投一票的權利。 股東可委託代表投票。我們持有的任何股份所附帶的投票權,只要是以國庫形式持有的,都將被暫停。然而,如果用益物權(VruchtgeBruik)或質押權在本公司收購普通股之前被授予,則另一人持有的股票的用益物權(VruchtgeBruik)的持有人以及我們持有的普通股的質押權的持有人並不排除他們可能必須對該等普通股進行投票的任何權利。我們不得就存在用益物權或質押權的 股票投票。 根據上述句子無權享有投票權的股票將不會被 用於確定參與投票的股東人數和出席或代表的股東人數,或已提供或代表的股本金額。 股東大會的決定以絕對多數票通過。除 荷蘭法律或公司章程規定的絕對多數或一致外。根據荷蘭法律和普遍接受的商業慣例,我們的公司章程並不提供一般適用於股東大會的法定人數要求。 在這方面,我們的做法與納斯達克上市規則第5620(C)條的要求不同,後者要求發行人在其章程中規定一般適用的法定人數,並且該 法定人數不得低於已發行有表決權股票的三分之一。 我們的董事會成員可以出席股東大會,他們在大會上扮演着顧問的角色。只要股份由我們持有,股份附帶的投票權就暫停。 我們在2023年召開了兩次股東大會。 2023年2月27日,召開了特別股東大會,任命史蒂夫·克羅格尼斯 為董事非執行董事,任期至2027年股東周年大會為止。 在2023年股東大會上,我們通過了2022財年的年度報告和年度賬目,再次任命J.唐納德·德貝蒂茲先生為董事董事會非執行董事,任期兩年,董事會被授權發行 股票,並授予認購我們股本中最多10%的股份的權利,認購範圍為會議日期和自 會議起18個月,並限制或排除有關此類(認購)股份的法定優先購買權,德勤會計師事務所被任命為公司2023財年的審計師是approved. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx年度報告2023年股東大會、投票權和准入|238 |
| Anti-Takeover Provisions Various protective measures are possible and permissible within the boundaries set by Dutch law and Dutch case law. We have not implemented specific measures with the aim of deterring takeover attempts. However, we have adopted several provisions that may have the effect of making a takeover of argenx more difficult or less attractive, including requirements that certain matters, including an amendment of our Articles of Association, may only be brought to our shareholders for a vote upon a proposal by our Board of Directors. No takeover bid has been instigated by third parties in respect of our equity during the current or previous fiscal years. Exchange Controls Under Dutch law, subject to the 1977 Sanction Act (Sanctiewet 1977) or otherwise by international sanctions, there are no exchange control restrictions on investments in, or payments on, shares (except as to cash amounts). There are no special restrictions in our Articles of Association or Dutch law that limit the right of shareholders who are not citizens or residents of the Netherlands to hold or vote shares. Amendments of Articles of Association The shareholders at a General Meeting may amend the Articles of Association, at the proposal of our Board of Directors, with the consent of the majority of the non-executive directors. A resolution by the shareholders at a General Meeting to amend the Articles of Association requires a simple majority of the votes cast in a meeting in which at least half of our issued and outstanding capital is present or represented, or at least two-thirds of the votes cast, if less than half of our issued and outstanding capital is present or represented at that meeting. Changing the rights of any of the shareholders will require the Articles of Association to be amended. The 2022 General Meeting approved the amendment of our current Articles of Association to align with current Dutch law and practice. The Articles of Association were amended pursuant to the notarial deed of partial amendment of the Articles of Association, executed on May 10, 2022. The full text of the Articles of Association and an unofficial English translation thereof are available on our website (www.argenx.com/investors). Transparency Directive We are a European public company with limited liability (Societas Europaea or SE) incorporated and existing under the laws of the Netherlands. The Netherlands is our EU home member state (lidstaat van herkomst) for the purposes of Directive 2004/109/EC (as amended by Directive 2013/50/EU), or the Transparency Directive, as a consequence of which we are subject to the DFSA in respect to certain ongoing transparency and disclosure obligations. In addition, as long as our shares are listed on Euronext Brussels and the ADSs on Nasdaq, we are required to disclose any regulated information which has been disclosed pursuant to the DFSA as well as in accordance with the Belgian Law of May 2, 2007, the Belgian Royal Decree of November 14, 2007 as well as Nasdaq Listing Rules. We must publish our annual accounts 4.6 4.7 4.8 4.9 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Anti-Takeover Provisions | 239 |
| within four months after the end of each financial year and our half-yearly figures within two months after the end of the first six months of each financial year. Within five calendar days after adoption of our annual accounts, we must file our adopted annual accounts with the AFM. Pursuant to the DFSA, we will be required, among other things, to make public without delay any change in the rights attaching to our shares or any rights to subscribe our shares. Dutch Financial Reporting Supervision Act Pursuant to the Dutch Financial Reporting Supervision Act (Wet toezicht financiële verslaggeving), the AFM supervises the application of financial reporting standards and has an independent right to (i) request an explanation from the Company regarding its application of the applicable financial reporting standards if, based on publicly known facts or circumstances, it has reason to doubt that the issuer’s financial reporting meets such standards and (ii) make a notification to the Company that its financial reports do not meet the applicable financial reporting standards, which notification may be accompanied by a recommendation to the Company to issue a press release on the subject matter. If the Company does not comply with such a request or recommendation, the AFM may request the Enterprise Chamber of the Court of Appeal in Amsterdam (Ondernemingskamer van het Gerechtshof te Amsterdam) to order the Company to (a) provide an explanation regarding its application of the applicable financial reporting standards to its financial reports or (b) prepare its financial reports in accordance with the Enterprise Chamber of the Court of Appeal’s instructions. This Annual Report also concerns the annual financial reporting within the meaning of 5:25c(2) DFSA. Dividends and Other Distributions Pursuant to Dutch law and the Articles of Association, the distribution of profits will take place following the adoption of our annual accounts, from which we will determine whether such distribution is permitted. We may only make distributions to the shareholders, whether from profits or from its freely distributable reserves, only insofar as its shareholders’ equity exceeds the sum of the paid-up and called-up share capital plus the reserves required to be maintained by Dutch law. The shareholders at the General Meeting may determine which part of our profits will be added to the reserves in consideration of our reserves and dividends policy. The remaining part of the profits after the addition to the reserves will be at the disposal of the shareholders at the General Meeting. Distributions of dividends will be made pro rata to the nominal value of each share. Subject to Dutch law and the Articles of Association, our board of directors, with the consent of the majority of the non-executive directors, may resolve to distribute an interim dividend if it determines such interim dividend to be justified by our profits. For this purpose, our board of directors must prepare an interim statement of assets and liabilities. Such interim statement shall show our financial position not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. An interim dividend can only be paid if (a) an interim statement of assets and liabilities is drawn up showing that the funds available for distribution are sufficient, and (b) our shareholders’ 4.10 4.11 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Dutch Financial Reporting Supervision Act | 240 |
| 股本超過已繳足股本和催繳股本的總和以及荷蘭法律規定的準備金。 經大多數非執行董事同意,我們的董事會可以 決議,根據我們對準備金和股息政策的適當遵守,我們從一個或多個可自由分配的準備金中向股東進行分配,而不是以利潤分配的方式。任何此類分配將按每股面值按比例分配。 股息和其他分配應不遲於董事會決定的日期 支付。自支付股息或分配之日起五年內未支付的股息和其他分配的債權將失效,任何此類金額將被視為已被沒收。 我們的董事會已宣佈了一系列中期分配,以支付向既有RSU持有人發行的所有此類股票的 總面值,並根據我們的股權激勵計劃 公司的可自由分配準備金。根據荷蘭法律,我們的董事會編制並提交了一份中期簡化資產負債表,證明有足夠的可自由分配儲備用於此類中期分配。這種臨時的簡化資產負債表是在荷蘭貿易登記處備案的。在2023年,這些中期分派的總金額約為6,600歐元(7,300美元)。 除了這些中期分派外,我們沒有就我們的普通股支付或宣佈任何現金股息,我們預計在可預見的 未來不會支付任何現金股息。我們所有的流通股都有相同的股息權。我們打算保留所有 可用資金和未來的任何收益,為我們業務的發展和擴張提供資金。 即使未來的運營會帶來可觀的可分配利潤,我們目前也打算將任何收益再投資於我們的業務,並且在我們有了支持持續現金股息的收入流之前,不會支付現金股息 。此外,未來向股東支付的任何股息將取決於股東大會的批准 根據我們董事會的提議,該提議 將在考慮到包括我們的業務前景、現金需求、財務 業績和新產品開發在內的各種因素後,得到大多數非執行董事的批准。 我們的網站上提供的公司章程包含了第20條中關於利潤分配的條款(利潤,分配和虧損)。 在發生清算的情況下獲得盈餘權 清償所有債務和清算成本後的任何剩餘將按股東所持股份的面值按比例分配給股東。 4.12 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息{Br}argenx年度報告2023年在清算情況下獲得盈餘的權利|241 |
| Material Modifications to the Rights of Security Holders and Use of Proceeds On July 18, 2023, we entered into an Underwriting Agreement with J.P. Morgan Securities LLC, as representatives of the several underwriters named therein, relating to a global offering of an aggregate of 2,244,899 ordinary shares of the Company, with nominal value €0.10 per share, including ordinary shares represented by ADSs, comprised of (i) 1,580,981 ADSs at a public offering price of $490.00 per ADS in the U.S. and countries outside the EEA, and (ii) 663,918 ordinary shares at an offering price of €436.37 per ordinary shares in a concurrent private placement in the EEA to certain legal entities all of which are qualified investors within the meaning of Regulation 2017/1129 of the European Parliament and of the Council of June 14, 2017, as amended. The offering was made pursuant to our effective shelf registration statement on Form F-3ASR (File No. 333-258251) filed on July 29, 2021, as supplemented by a preliminary prospectus supplement dated July 17, 2023, filed with the SEC on July 17, 2023, and a final prospectus supplement dated July 18, 2023, filed with the SEC on July 20, 2023. The offering closed on July 24, 2023. In connection with this offering, we granted the underwriters a 30-day option to purchase up to 336,734 additional ordinary shares (which may be represented by ADSs), which was exercised in full. The net proceeds to us from the sale of the ADSs and ordinary shares in this offering, after deducting the underwriting discounts and commissions and estimated offering expenses payable by the Company, was $1.2 billion (€1.1 billion). The offering closed on July 24, 2023. None of the underwriting discounts and commissions or offering expenses were paid to directors, officers or general partners of ours or their associates or to persons owning 10% or more of any class of our equity securities, or to any of our affiliates. We have not used any of the net proceeds from the offering to make payments, directly or indirectly, to any director, officer or general partner of ours or to their associates, persons owning 10% or more of any class of our equity securities, or to any of our affiliates. We have invested the net proceeds from the offering in cash and cash equivalents and current financial assets. There has been no material change in our planned use of the net proceeds from the offering as described in our final prospectus supplement filed pursuant to Rule 424(b)(5) under the Securities Act with the SEC on July 20, 2023 (File No.333-258251). The registration statement was effective on July 29, 2021. Enforcement of civil liabilities We are a European public company with limited liability (Societas Europaea or SE) incorporated under the laws of the Netherlands. Substantially all of our assets are located outside the U.S. The majority of our directors reside outside the U.S. As a result, it may not be possible for investors to effect service of process within the U.S. upon such persons or to enforce against them or us in U.S. courts, including judgments predicated upon the civil liability provisions of the federal securities laws of the U.S. The U.S. and the Netherlands currently do not have a treaty providing for the reciprocal recognition and enforcement of judgments, other than arbitration awards, in civil and commercial matters. Consequently, a final judgment for payment given by a court in the U.S., whether or not predicated solely upon U.S. securities laws, would not automatically be recognized or enforceable in the Netherlands. In order to obtain a judgment which is enforceable in the Netherlands, the party in whose favor a final and conclusive judgment of the U.S. court has been rendered will be required to file its claim with a court of competent 4.13 4.14 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Modifications to the Rights of Security Holders and Use of Proceeds | 242 |
| 荷蘭的司法管轄權。該方當事人可向荷蘭法院提交美國法院作出的最終判決。本法院在評估相關美國法院作出的判決時將有一定程度的自由裁量權。根據荷蘭最高法院的判例法,荷蘭法院原則上必須使該法院關於合同義務的最終和可執行的判決具有決定性效力,而不對所裁決的實體事項進行復審或重新訴訟,前提是:(I)所涉美國法院根據國際公認的理由接受管轄權以接受管轄權,(Ii)在該法院進行的訴訟符合正當程序原則(應按正當程序進行分拆),(Iii)該判決不違反荷蘭的公共政策,以及(Iv)該判決與荷蘭法院在同一當事人之間作出的判決或外國法院在同一事項爭議中基於相同訴訟理由作出的先前判決不相牴觸,只要該先前判決滿足使其在荷蘭具有約束力所需的條件。荷蘭法院可能會 拒絕承認和執行懲罰性賠償或其他不符合荷蘭法律秩序的裁決。此外,荷蘭法院可以減少美國法院給予的損害賠償金額,並僅在補償實際損失或損害賠償所必需的範圍內才承認損害賠償。美國法院在荷蘭的判決的執行和承認僅受《荷蘭民事訴訟法典》的規定管轄。 執行美國法院判決的原始訴訟或與美國聯邦或州證券法的民事責任條款有關的訴訟不能在比利時直接執行 。美國和比利時目前沒有條約規定在民事和商事事務中相互承認和執行判決,但仲裁裁決除外。因此,由美國法院作出的最終付款判決,無論是否完全基於美國證券法,都不會自動在比利時得到承認或強制執行。為了使 美國法院基於民事責任支付款項的最終判決在比利時領土上產生任何影響, 要求該判決得到比利時法院的承認,並宣佈可由比利時法院根據《個人利益法》的相關規定執行。承認或執行並不意味着審查案件的是非曲直,也不涉及任何互惠要求。但是,如果美國的判決 違反了《公民權利公約》第25條中詳盡列出的一項或多項駁回理由,則該判決將不會在比利時獲得承認或宣佈可強制執行。除了承認或執行之外,美國聯邦 或州法院針對我們的判決也可以作為比利時 法院類似訴訟的證據,如果它符合根據作出判決的州的法律 所要求的判決真實性的條件。此外,關於在比利時通過法律程序強制執行(包括承認在比利時的外國法院判決),債務人應按判決金額的3%繳納登記税,如果比利時法院或外國法院命令債務人支付的金額超過12,500歐元(一)可自動強制執行並在比利時登記,或(二)由比利時法院強制執行。登記税由債務人支付。 債務人有責任按照命令付款或清算的決定或確定債權人優先順序的決定確定的比例繳納登記税。債務人(S)被責令連帶支付的,承擔連帶責任。自比利時法院作出的執行判決的第二份認證副本起支付印花税,最高金額為1,450歐元。 荷蘭和比利時民事訴訟程序在許多方面與美國民事訴訟程序有很大不同。就舉證而言,美國法律和基於普通法的其他幾個司法管轄區的法律規定了審前證據開示,通過這一程序,訴訟當事人可以在庭審前強迫不利的 或第三方出示文件和證人的證詞。通過這種方式獲得的證據可能會be ar Gr g oup enx Factors Risk Go Corporate vernance資本 分享財務 審查報表 財務非財務 信息 Argenx 2023年年度報告民事責任的執行情況|243 |
| 對任何訴訟的結果都具有決定性。根據荷蘭或比利時法律, 不存在此類審前證據開示程序。 在符合上述規定並根據適用條約送達程序的前提下,投資者 可能能夠在荷蘭或比利時執行從美國聯邦或州法院獲得的民事和商事判決。但是,不能保證 這些判決是可執行的。此外,荷蘭或比利時法院 是否會接受司法管轄權並對在荷蘭或比利時啟動的僅以美國聯邦證券法為基礎的原始訴訟施加民事責任還值得懷疑。 控制和程序 披露控制和程序 我們的管理層在首席執行官和首席財務官的參與下,評估了截至2023年12月31日,我們的披露控制和程序的設計和運作的有效性。雖然 任何披露控制系統和程序的有效性都有固有的侷限性,包括人為錯誤的可能性以及規避或推翻控制和程序的可能性,但我們的披露控制和程序旨在為實現其目標提供 合理的保證。 根據我們的評估,截至2023年12月31日,我們的首席執行官和首席財務官得出結論,根據交易法第13a-15(E)條,披露控制和程序,(I)在合理保證的水平上有效,以確保根據交易法提交或提交的報告中要求披露的信息在委員會規則和表格規定的時間內被記錄、處理、彙總和報告,並且(Ii)在合理保證的水平上有效,以確保在根據交易法提交或提交的報告中披露的信息被累積並傳達給我們公司的管理層,包括我們的首席執行官和首席財務官,允許 及時做出有關要求披露的決定。 管理層年度報告財務報告控制 我們的管理層負責建立和維護對財務報告的充分內部控制 規則13a-15(F)和規則15d-15(F)中定義了該術語。我們對財務報告的內部控制是在我們的首席執行官和首席財務官的監督下設計的,旨在根據國際會計準則理事會發布的國際會計準則,為財務報告的可靠性和為外部報告目的編制財務報表提供合理的 保證。 我們對財務報告的內部控制包括與以下方面有關的政策和程序: 保持合理詳細、準確和公平地反映交易和資產處置的記錄,提供合理保證,以允許根據國際財務報告準則編制財務報表所需的方式記錄交易,並且僅根據管理層和董事的授權進行收支,並提供關於防止或及時發現任何未經授權的收購的合理保證。使用或 處置可能對我們的合併財務報表產生實質性影響的資產 4.15 4.15.1 4.15.2 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年年度報告控制和程序|244 |
| 由於其固有的侷限性,財務報告的內部控制可能無法防止或 發現所有錯誤陳述。此外,對未來期間的內部控制有效性的任何評估預測都可能面臨這樣的風險:由於條件的變化,控制可能變得不充分 ,政策或程序的遵守程度可能會惡化。 我們的管理層已根據特雷德韋委員會(COSO)贊助 組織委員會於2013年發佈的內部控制綜合框架評估了財務報告內部控制的有效性。基於這一評估,我們的 管理層得出結論,截至2023年12月31日,我們對財務報告的內部控制是有效的。 註冊公眾 會計師事務所 我們截至2023年12月31日的財務報告內部控制的有效性已由我們的獨立註冊會計師事務所德勤會計師事務所進行審計。 他們的審計報告包含在本 年報所包括的經審計的合併財務報表中。 在本年度報告涵蓋的期間內,財務內部控制的變化 報告 我們沒有對我們的財務報告內部控制進行任何重大影響,或很可能對我們的財務報告內部控制產生重大影響。 2024年5月7日在荷蘭阿姆斯特丹召開的2024年財務日曆2024年5月9日第一季度財務業績 2024年7月25日、2024年半年和2024年第二季度財務業績 10月31日2024年第三季度財務業績 4.15.3 4.15.4 4.16 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 2023年年度報告財務日曆2024年|245 |
| 經營和財務回顧 和展望 《經營和財務回顧與展望》應與本年度報告其他部分包括的財務報表和相關附註中的信息 一起閲讀。以下討論基於我們根據國際財務報告準則和國際會計準則委員會發布的解釋編制的財務信息,該解釋由歐盟通過的國際財務報告解釋委員會(EU-IFRS)通過,並符合《國際財務報告準則》第2冊第9部分的法律要求。以下討論包括涉及風險、不確定性和 假設的前瞻性陳述。由於許多因素,我們的實際結果可能與這些前瞻性陳述中預期的結果大不相同,這些因素包括但不限於 “風險因素”部分和本年度報告其他部分中描述的那些因素。見本年度報告中的“前瞻性陳述”。 5.1概述247 5.2列報基礎249 5.3資本化和負債253 5.4關鍵會計估計和判斷254 5.5業務結果255 5.6流動性和資本資源260 5.7研究與開發,專利和許可263 5.8趨勢信息264 5.9表外安排264 5.10合同義務264 5.11關於獨立審計師264 5.12重大合同和關聯方交易的信息265 5.13員工268 5.14保險268 5.15法律和仲裁程序269 5.16税務269 5年度報告2023年財務回顧|246 |
| 5 Operating and Financial Review and Prospects Overview Since our inception in 2008, we have focused most of our financial resources and efforts towards developing our SIMPLE Antibody™ Platform and antibody engineering technologies, identifying potential product candidates, establishing process, development and manufacturing capabilities for our product candidates and advancing multiple discovery programs into the clinic. In 2022, we executed on our global launch of VYVGART our first-in-class neonatal FcRn blocker for intravenous use, which is now approved in the U.S., Japan, Europe, Israel, Canada and China for gMG. In 2023, we executed on our global launch of VYVGART SC, the first-and-only neonatal FcRn blocker administered by subcutaneous injection, which is now approved in the U.S. and Europe. In 2023, the successful commercialization of VYVGART and VYVGART SC generated a global product net sales of $1.2 billion. On our research and development, we continue towards advancing a deep pipeline of both clinical- and preclinical-stage product candidates for the treatment of severe autoimmune diseases. Leveraging our technology suite and clinical expertise, we have advanced several candidates into late-stage clinical development and we currently have multiple programs in the discovery stage. As of December 31, 2023 and December 31, 2022, we had cash, cash equivalents and current financial assets of $3,180 million and $2,193 million, respectively. Our Statement of financial position shows our total assets of $4,542 million for the year ended December 31, 2023, compared to $3,134 million for the year ended December 31, 2022. The main reason for the material change in balance sheet total are the various equity financing rounds, completed over the periods covered by the financial statements. Since our inception, we have incurred significant operating losses. For the years ended December 31, 2023 and 2022, we incurred total comprehensive losses of $295 million and $730 million, respectively. As of December 31, 2023, we had accumulated losses of $2,405 million. Although we have generated revenue of $1.2 billion from global product net sales of VYVGART and VYVGART SC for gMG in the fiscal year ended December 31, 2023, we can provide no assurances that we will be able to achieve or sustain profitability based on product net sales in that indication alone or that we will be able to receive regulatory approval of and commercialize VYVGART or VYVGART SC in other indications or in other countries. On December 17, 2021, the FDA approved efgartigimod, which is marketed as VYVGART, for the intravenous treatment of gMG in adult patients who are AChR-AB+, followed by Japanese PMDA approval (including seronegative patients) and approval the EU Commission in 2022 and China’s NMPA approval on July 30, 2023. On June 20, 2023, the FDA approved VYVGART SC for the subcutaneous treatment of gMG in adult patients who are AChR-AB+, followed by approval of the EU Commission on November 16, 2023. These are the only approved products we currently have. 5.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Overview | 247 |
| We expect our expenses to continue to increase as we expand our global commercial infrastructure and drug product inventory for VYVGART and VYVGART SC for the treatment of gMG, the commercial launch of VYVGART SC for the treatment of CIDP if and when approval obtained, the advancement of our clinical-stage pipeline, including ongoing registrational clinical trials across five indications of efgartigimod, and continued investment in our IIP. We anticipate that our expenses will increase if and as we: Research and development activities: · execute the phase 2 clinical trials of efgartigimod in SjD, POTS post-COVID-19 and AMR · execute the phase 2 clinical trials with our partner Zai Lab in MN and LN · execute the seamless phase 2/3 clinical trials of efgartigimod in Myositis and BP · execute the phase 3 clinical trials of efgartigimod in MG seronegatives and Pediatric and TED · launch phase 2 and/or phase 3 in other indications with efgartigimod · execute the phase 2 clinical trials of empasiprubart in MMN, DGF and DM · execute the phase 1 clinical trial of ARGX-119 in healthy volunteers and the phase 1b / phase 2a clinical trials in CMS and ALS, respectively · continue the research and development of our other clinical- and preclinical-stage product candidates and discovery stage programs; and · seek regulatory approvals for any product candidates, including new indications, that successfully complete clinical trials. Pre-commercial and commercial activities: · further build our sales, marketing and distribution infrastructure and scale-up of manufacturing capabilities for the commercialization expansion of VYVGART and VYVGART SC and any product candidate, including new indications, for which we may obtain approval; and · expand our global reach enabling us to commercialize any product candidates, including new indications, for which we may obtain regulatory approval. Other activities: · seek to enhance our technology platform and discover and develop additional product candidates; · maintain, expand and protect our intellectual property portfolio, including litigation costs associated with defending against alleged patent infringement claims; · add clinical, scientific, operational, financial and management information systems and personnel, including personnel to support our product development and potential future commercialization efforts; and · experience any delays or encounter any issues, including failed studies, ambiguous clinical trial results, safety issues or other regulatory challenges. We expect that the costs of development and commercialization might also increase due to current and future collaborations with research and development partners as well as commercial partners. Information pertaining to the year ended December 31, 2022 was included in our annual report on Form 20-F for the year ended December 31, 2022 under Item 5, “Operating and Financial Review and Prospects,” which was filed with the SEC on March 16, 2023. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Overview | 248 |
| 列報基礎 外幣交易 職能貨幣和列報貨幣 各實體合併財務報表中所列項目使用實體所處經濟環境的貨幣進行估值。合併的 財務報表以美元(美元)列報,這是公司的列報貨幣。 銷售產品的收入 銷售貨物的收入以反映公司預期有權獲得的對價的金額確認,以換取將貨物轉讓給 客户,當客户獲得對所交付貨物的控制權時,這意味着客户有能力指示資產的使用。在與客户簽訂的合同中, 承諾的對價可以包括固定金額、可變金額或兩者兼有。對價金額可能會因折扣、返點、退貨、 退款或其他類似項目而有所不同。當確認的收入金額極有可能不會受到未來重大逆轉的影響時,或有對價計入交易價格中。 我們的產品淨銷售額主要包括VYVGART在美國、日本、歐洲和中國的銷售以及VYVGART SC在美國和歐洲的銷售。根據國際財務報告準則第15號“與客户簽訂的合約所得收入”,當本公司在某一時間點符合收入確認準則下的履約責任時,產品淨銷售額即予確認。 VYVGART及VYVGART SC的商業銷售收入於綜合財務報表中附註15“產品淨銷售額”列示。根據國際財務報告準則15“與客户簽訂合同的收入”,此類收入在產品實際轉讓時根據與客户商定的交付和驗收條款確認。交易價格在客户 獲得商品的合法所有權時支付。 協作和許可協議的收入 迄今的收入主要包括與協作和 許可協議相關的里程碑、許可費、不可退還的預付費用和研發服務費。 當客户獲得對承諾的商品或服務的控制權時,我們確認收入,其金額反映了我們希望從這些商品和服務交換中獲得的對價。為了確定我們確定 在IFRS 15範圍內的協議的收入確認,我們遵循了IFRS 15五步模型。公司目前在IFRS 15的範圍內有兩個正在進行的協作和許可協議: 5.2 5.2.1 5.2.2 5.2.3 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx年度報告2023年列報基礎|249 |
| Zai Lab For the collaboration agreement with Zai Lab the Company has assessed that there is more than one distinct performance obligation, being the transfer of a license and supply of clinical and commercial product. The Company concluded that these performance obligations are distinct in the context of the contract. Therefore, the Company allocates the transaction price to all performance obligations identified. The transaction price of the agreement is composed of (i) a fixed part, that being an upfront payment in the form of newly issued Zai Lab shares, and a guaranteed, non-creditable, non-refundable payment and (ii) a milestone payment for approval of efgartigimod in the U.S. and the consideration received in return for the supply of clinical and commercial product. The fixed part of the transaction price, as well as the milestone for approval of efgartigimod in the U.S. has been allocated to the transfer of a license performance obligation. The Company concluded that the license as of the effective date of the contract, being January 2021, has standalone value. As such, the Company concluded that the promise in granting the license to Zai Lab is to provide a right to use the entity’s intellectual property as it exists at the point in time at which the license is granted and therefore, revenue was recognized at a point in time. Under the collaboration agreement, the Company provides clinical and commercial supply to Zai Lab. Company concludes to recognize such sales as revenue given that the Company acts as principal in the transaction as the risk related to inventory is born by the Company until the inventory is transferred to Zai Lab. The revenue related to clinical supply is recorded under line item “Collaboration revenue”. The revenue related to commercial supply is recorded under line item “product net sales” in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss). The income related to royalties is recorded under line item “Collaboration revenue”. AbbVie For the collaboration agreement with AbbVie the Company has determined that the transfer of license combined with the performance of research and development activities represent one single performance obligation. The Company concluded that the license is not distinct in the context of the contract. The transaction price is composed of a fixed part, that being an upfront license fee, and a variable part, being milestone payments and cost reimbursements of research and development activities delivered. Milestone payments are only included in the transaction price to the extent it is highly probable that a significant reversal in the amount of cumulative revenue recognition will not occur when the uncertainty associate with the variable consideration is subsequently resolved. Management estimates the amount to be included in the transaction price upon achievement of the milestone event. Sales-based milestones and sales-based royalties are a part of the Company’s arrangements but are not yet included in its revenues. The transaction price has been allocated to the single performance obligation and revenues has been recognized over the estimated service period based on an input model, being the percentage of completion method. The upfront license fee has been fully recognized since 2021 as the performance obligation has been fulfilled at that time. Milestone payments that become highly probable after the performance obligation has been fulfilled are therefore recognized at that point in time. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Basis of Presentation | 250 |
| Research and development Expenses Research and development expenses consist principally of: · external research and development expenses related to (i) chemistry, manufacturing and control costs for our product candidates, both for preclinical and clinical testing, all of which is conducted by specialized contract manufacturers, (ii) fees and other costs paid to CROs in connection with preclinical testing and the performance of clinical trials for our product candidates, (iii) costs associated with regulatory submissions and approvals, QA and pharmacovigilance and (iv) costs associated with post-approval clinical trails. · personnel expense related to compensation of research and development staff and related expenses, including salaries, benefits and share‑based payment expenses; · materials and consumables expenses; · depreciation and amortization of tangible and intangible fixed assets used to develop our product candidates; and · IT expenses; · other expenses including, but not limited to costs associated with obtaining and maintaining patents and other intellectual property. We incur various external expenses under our collaboration and license agreements for material and services consumed in the discovery and development of our partnered product candidates. Our research and development expenses may vary substantially from period to period based on the timing of our research and development activities, including the timing of the initiation of clinical trials, production of product batches and enrolment of patients in clinical trials. Research and development expenses are expected to increase as we advance the clinical development of efgartigimod and empasiprubart and further advance the research and development of our other early-stage pipeline candidates. The successful development of our product candidates is highly uncertain. At this time, we cannot reasonably estimate the nature, timing and estimated costs of the efforts that will be necessary to complete the development of, or the period, if any, in which material net cash inflows may commence from any of our product candidates. This is due to numerous risks and uncertainties associated with developing drugs, as fully described in Item 3.D. “Risk Factors,” and including the uncertainty of: · the scope, rate of progress and expense of our research and development activities; · the successful enrollment in, and completion of clinical trials; · the ability to market, commercialize and achieve market acceptance for efgartigimod or any other product candidate that we may develop in the future, if approved; · establishing and maintaining a continued acceptable safety profile for our product candidates; · the terms, timing and receipt of regulatory approvals from applicable regulatory authorities; · the successful completion of preclinical studies necessary to support IND applications in the U.S. or similar applications in other countries; · the expense of filing, prosecuting, defending and enforcing patent claims and other intellectual property rights and our current and future collaborators continuing their collaborations with us. 5.2.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Basis of Presentation | 251 |
| Selling, general and administrative Expenses Selling, general and administrative expenses consist primarily of: · personnel expenses relating to salaries and related costs for personnel, including share-based compensation, of our employees in executive, finance, business development, marketing, commercial and support functions; · professional fees for business development, marketing, IT, audit, commercial, legal services and investor relations costs; · Board of Directors expenses consisting of directors’ fees, travel expenses and share-based compensation for non-executive board members; · costs associated with commercial launch of VYVGART and VYVGART SC for the treatment of gMG and marketing and promotional activities, pre-launch activities of VYVGART and VYVGART SC in other indications and continued investment in supply chain and costs associated with pre-launch activities in other indications; · allocated facilities costs; and · other Selling, general and administrative expenses, including leasing costs, office expenses, travel costs. We expect our general and administrative expenses to increase as we continue to support our growth. Such costs include increases in our personnel, additional IT-related expenses, and expenses and costs associated with compliance with the regulations governing public companies. We expect our selling and marketing expenses to increase due to marketing and promotional activities with respect to the ongoing commercial launch of VYVGART, VYVGART SC and preparation of commercial launch of our other product candidates. Financial Income (Expense) Financial income mainly reflects interest earned on our cash and cash equivalents and current financial assets and net gains on our cash and cash equivalents and current financial assets held at fair value through profit or loss. Financial expense corresponds mainly to net losses on cash and cash equivalents and current financial assets held at fair value through profit or loss. Exchange Gains (Losses) Our exchange gains (losses) relate to (i) our transactions denominated in foreign currencies, mainly in euro, and which generate exchange gains or losses and (ii) the translation at the reporting date of assets and liabilities denominated in foreign currencies into USD, which is our functional and presentation currency. For more information on currency exchange fluctuations on our business, please see Note 26 “Financial instruments and financial risk management – Foreign exchange risk” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2023. We have no derivative financial instruments to hedge interest rate and foreign currency risk. 5.2.5 5.2.6 5.2.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Basis of Presentation | 252 |
| Income Tax Expense We have a history of losses in certain jurisdictions, including Belgium and the Netherlands. We may continue to incur losses as we continue to invest in our clinical and pre-clinical development programs and our discovery platform, and we incur costs for various commercial launches and regulatory approvals. Consequently, we do not recognize any deferred tax asset regarding certain tax attributes on our consolidated statements of financial position. We incur current income tax expense and recognize deferred tax assets in various subsidiaries in view of the transfer pricing policy set up between argenx BV and these subsidiaries. For more information on income tax and deferred tax, please see Note 25 “Income tax expense” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2023. Capitalization and Indebtedness The table below sets forth our capitalization as of December 31, 2023 on an actual basis: (in thousands of $) As of December 31, 2023 (audited) Total current debt (including current portion of non-current debt) – Guaranteed – Secured – Unguaranteed/unsecured – Total non-current debt (excluding current portion of non-current debt) – Guaranteed – Secured – Unguaranteed/unsecured – Shareholder equity 4,097,507 Share capital 7,058 Share premium 5,651,497 Legal reserve(s) 1) 131,543 Retained earnings (2,404,844) Other reserves 712,253 Total 4,097,507 1) Legal reserves are the amount of translation differences. The table below sets forth our indebtedness as of December 31, 2023 on an actual basis: 5.2.8 5.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Capitalization and Indebtedness | 253 |
| (in thousands of $) As of December 31, 2023 (audited) A. Cash 20,744 B. Cash equivalents 1) 2,028,100 C. Other current financial assets 2) 1,131,000 D. Liquidity (A + B + C) 3,179,844 E. Current financial debt (including debt instruments, but excluding current portion of non-current financial debt) – F. Current portion of non-current financial debt 3) 4,646 G. Current financial indebtedness (E + F) 4,646 H. Net current financial indebtedness (G – D) (3,175,198) I. Non-current financial debt (excluding current portion and debt instruments) 3) 15,354 J. Debt instruments – K. Non-current trade and other payables – L. Non-current financial indebtedness (I + J + K) 15,354 M. Total financial indebtedness (H + L) (3,159,844) 1) See note 11 “Cash and cash equivalents” to our consolidated financial statements in section “Consolidated Financial Statements”. 2) See note 10 “Financial assets – current” to our consolidated financial statements in section “Consolidated Financial Statements”. 3) Please note that financial debt balances as presented in the table above do not include any indirect or contingent indebtedness. For more information on the Company’s indirect and contingent indebtedness, please see note 29 “Commitments” to our consolidated financial statements in section “Consolidated Financial Statements”. As of December 31, 2023, current financial debt (as disclosed in item E. in the table above) included current liabilities related to short-term leases in the amount of $4.6 million and non-current financial debt (as disclosed in item I. in the table above) included non-current liabilities related to long-term leases in the amount of $15.4 million. More information is included in our consolidated financial statements and related notes included in section “Consolidated Financial Statements” Critical Accounting Estimates and Judgments In the application of the Company’s accounting policies, which are described above, the Company is required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods. 5.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Critical Accounting Estimates and Judgments | 254 |
| Critical estimates in applying accounting policies Gross to net adjustments The product gross sales are subject to various deductions, which are primarily composed of rebates to government agencies, distributors, health insurance companies and managed healthcare organizations. These deductions represent estimates of the related obligations, requiring the use of judgment when estimating the effect of these sales deductions on product gross sales for a reporting period. These adjustments are deducted from product gross sales to arrive at product net sales. The significant components of variable consideration under revenue recognition policy summarizes the nature of these deductions and how the deduction is estimated, see Note 2.17 “Product net sales”. After recording these, product net sales represent the Company’s best estimate of the cash that we expect to ultimately collect. If in future periods the actuals vary from prior period best estimates, this would affect revenue in the period of adjustment. Results of Operation Comparison of Years Ended December 31, 2023 and 2022 Year Ended December 31, (in thousands of $) 2023 2022 % Change Product net sales 1,190,783 400,720 197% Collaboration revenue 35,533 10,026 254% Other operating income 42,278 34,520 22% Total operating income 1,268,594 445,267 185% Cost of sales (117,835) (29,431) 300% Research and development expenses (859,492) (663,366) 30% Selling, general and administrative expenses (711,905) (472,132) 51% Loss from investment in joint venture (4,411) (677) 552% Total operating expenses (1,693,643) (1,165,607) 45% Operating loss (425,049) (720,340) (41)% Financial income 107,386 27,665 288% Financial expense (906) (3,906) (77)% Exchange gains/(loss) 14,073 (32,732) (143)% Loss for the year before taxes (304,496) (729,314) (58)% Income tax benefit 9,443 19,720 (52)% Loss for the year (295,053) (709,593) (58)% Weighted average number of shares outstanding 57,169,253 54,381,371 Basic and diluted (loss) per share (in $) (5.16) (13.05) 5.4.1 5.5 5.5.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Results of Operation | 255 |
| Product net sales Year Ended December 31, (in thousands of $) 2023 2022 United States 1,046,592 377,659 Japan 56,432 15,764 EMEA 72,852 7,297 China 14,907 – Total product net sales 1,190,783 400,720 For the twelve months ended December 31, 2023, the product net sales were mainly related to sales of VYVGART in the U.S., Japan, EU and China and VYVGART SC in the U.S. and Europe. Year Ended December 31, (in thousands of $) 2023 2022 2021 Product gross sales 1,342,148 446,923 – Gross to net adjustment (151,365) (46,203) – Product net sales 1,190,783 400,720 – Collaboration Revenue Year Ended December 31, (in thousands of $) 2023 2022 % Change AbbVie 30,000 – N/A% Other – 5,365 (100)% Milestone payments 30,000 5,365 459% Other – 424 (100)% Research and development service fees – 424 (100)% Zai Lab 5,533 4,238 31% Other collaboration revenues 5,533 4,238 31% Total collaboration revenue 35,533 10,026 254% Our collaboration revenue increased by $26 million to $36 million for the year ended December 31, 2023, compared to $10 million for the year ended December 31, 2022. The collaboration revenue recognized in the year ended December 31, 2023 was mainly the result of the recognition of a $30 million development milestone related to the AbbVie collaboration agreement. The revenue recognized during the year ended December 31, 2022, from milestone payments primarily relates to €5 million triggered by the option exercised by LEO Pharma to enter into the LEO Pharma Collaboration Agreement for ARGX-112. The increase in revenue recognition from “Other collaboration revenues” of $1 million was primarily driven by the royalties on net sales of VYVGART in Greater China through Zai Lab. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Results of Operation | 256 |
| Other Operating Income Year Ended December 31, (in thousands of $) 2023 2022 % Change Grants 2,538 2,186 16% Research and development incentives 27,815 19,502 43% Payroll tax rebates 11,925 8,576 39% Change in fair value on non-current financial assets – 4,256 (100)% Total 42,278 34,520 22% Other operating income increased by $8 million to $42 million for the year ended December 31, 2023, compared to $35 million for the year ended December 31, 2022. The $8 million increase was primarily driven by: · the increase in research and development incentives due to a Belgian research and development tax incentive scheme, as a result of the overall increased research and development costs incurred. · the increase in payroll tax rebates for the year ended December 31, 2023, as a result of higher research and development personnel expenses eligible for rebates for the year ended December 31, 2023; and · a decrease of $4 million due to the fact that there was no change in fair value on our profit share in AgomAb for the year ended December 31, 2023; For more information regarding governmental policies that could affect our operations, see “Business Overview” and “Healthcare Law and Regulation.” Research and Development Expenses Year Ended December 31, (in thousands of $) 2023 2022 % Change Personnel expense 226,344 162,010 40% External research and development expenses 483,192 366,955 32% Materials and consumables 4,057 2,396 69% Depreciation and amortization 105,546 102,132 3% IT expenses 19,935 12,678 57% Other expenses 20,418 17,194 19% Total 859,492 663,366 30% Our research and development expenses totaled $859 million and $663 million for the years ended December 31, 2023 and 2022, respectively. The increase of $196 million in fiscal year 2023 as compared to fiscal 2022 is primarily driven by Personnel expense and External research and development expenses. Personnel expense primarily relates to internal and external personnel. The expense also includes share-based compensation expenses related to the grant of stock options and RSUs to our research and development employees. We employed on average 607 full-time equivalents in our research and development functions in the year ended December 31, 2023, compared to 475 in the year ended December 31, 2022. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Results of Operation | 257 |
| Our external research and development expenses for the year ended December 31, 2023 totaled to $483 million, compared to $367 million for the year ended December 31, 2022. The expense reflects clinical trial costs and manufacturing expenses related to the development of our product candidate portfolio. The table below provides additional detail on our external research and development expenses by program: Year Ended December 31, (in thousands of $) 2023 2022 % Change efgartigimod 361,676 280,572 29% cusatuzumab 14,298 13,554 5% empasiprubart 47,636 32,384 47% Other programs 1) 59,582 40,445 47% Total 483,192 366,955 32% 1) Other programs include general expenses not allocated to specific program of $27 million in 2023 and $23 million in 2022. External research and development expenses for our lead product candidate efgartigimod totaled $362 million for the year ended December 31, 2023, compared to $281 million for the year ended December 31, 2022. This increase corresponds primarily to manufacturing and clinical development activities in relation to: · the execution of two Phase 3 clinical trials in MG Ph3b and Pediatric · the execution of two Phase 3 clinical trials in CIDP; · the execution of two Phase 3 clinical trial in PV and PF; · the execution of Phase 2 and 3 clinical trials in BP, Myositis, LN, MN, AMR, POTS post-COVID-19, SjD, TED and AAV; · the execution of multiple Phase 2 clinical trials in empasiprubart in MMN, DGF and DM · the execution of one HV clinical trial in ARGX-119 · the execution of pre-clinical activities. External research and development expenses for empasiprubart totaled $48 million for the year ended December 31, 2023 compared to $32 million for the year ended December 31, 2022. This increase of $15 million was due to the ramp up of Ph2 clinical trials in MMN, DGF and DM and further investments in Discovery activities. External research and development expenses on other programs increased by $19 million to $60 million for the year ended December 31, 2023, compared to $40 million for the year ended December 31, 2022. Of the total external research and development expense, $27 million relates to general allocation of expenses. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Results of Operation | 258 |
| Selling, general and administrative Expenses Year Ended December 31, (in thousands of $) 2023 2022 % Change Personnel expenses 303,033 234,740 29% Marketing services 202,146 115,950 74% Professional fees 108,820 62,620 74% Supervisory board 8,362 6,912 21% Depreciation and amortization 2,366 2,211 7% IT expenses 20,408 17,431 17% Other expenses 66,770 32,268 107% Total Selling, general and administrative expenses 711,905 472,132 51% Our Selling, general and administrative expenses totaled $712 million and $472 million for the years ended December 31, 2023 and 2022, respectively. The increase in our Selling, general and administrative expenses for the year ended December 31, 2023 was principally resulting from: · increased professional and marketing fees, including promotional and marketing costs primarily due to the commercial launch of VYVGART and VYVGART SC; · increased costs of the salary and wages and benefits to our Selling, general and administrative employees due to planned increase in the headcount; · increased costs associated with additional employees recruited to strengthen our Selling, general and administrative activities, for the commercial launch of VYVGART and VYVGART SC; and · continued investment in our IT infrastructure; We employed on average 681 full-time equivalents in our Selling, general and administrative functions in the year ended December 31, 2023, compared to 442 in the year ended December 31, 2022. Financial income and (expense) For the year ended December 31, 2023, financial income amounted to $107 million compared to $28 million for the year ended December 31, 2022. The increase of $80 million in 2023 related primarily to higher interests. For the year ended December 31, 2023, financial expense amounted to $1 million compared to $4 million for the year ended December 31, 2022. Exchange Gains (Losses) Exchange gains totaled $14 million for the year ended December 31, 2023, compared to exchange losses of $33 million for the year ended December 31, 2022. The decrease was mainly attributable to unrealized exchange rate gains on the cash, cash equivalents and current financial assets position in euro during the year ended December 31, 2023 as compared to unrealized exchange rate losses on the cash, cash equivalents and current financial assets position during the year ended December 31, 2022. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Results of Operation | 259 |
| Liquidity and Capital Resources Sources of Funds Since our inception in 2008, we have invested most of our resources in developing our product candidates, building our intellectual property portfolio, developing our supply chain, conducting business planning, raising capital and providing general and administrative support for these operations. We currently have 2 products approved by the FDA and as of the year ended December 31, 2022, net product sales also started to contribute to the funding of our operations. To date, we have funded our operations through public and private placements of equity securities, upfront, milestone and expense reimbursement payments received from our collaborators, funding from governmental bodies and interest income from the investment of our cash, cash equivalents and financial assets. Through December 31, 2023, we have raised gross proceeds of $5.6 billion from private and public offerings of equity securities. We have made product net sales of $1.2 billion during the twelve months ended December 31, 2023. Our cash flows may fluctuate, are difficult to forecast and will depend on many factors. On December 31, 2023, we had cash, cash equivalents and current financial assets of $3,180 million, compared to $2,193 million on December 31, 2022. We have no ongoing material financing commitments, such as lines of credit or guarantees, that are expected to affect our liquidity over the next five years, other than leases and our commitments to Lonza and Fujifilm which are detailed in Note 29 “Commitments” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2023. For more information as to the risks associated with our future funding needs, see Item “Risk Factors – Risk Factors Related to argenx’s Financial Position and Need for Additional Capital.” For more information as to our financial instruments, please see Note 26 “Financial instruments and financial risk management” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2023. 5.6 5.6.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Liquidity and Capital Resources | 260 |
| Cash Flows Comparison for the Years Ended December 31, 2023 and 2022 The table below summarizes our cash flows for the years ended December 31, 2023 and 2022. Year Ended December 31, (in thousands of $) 2023 2022 Variance Cash and cash equivalents at beginning of the period 800,740 1,334,676 (533,936) Net cash flows (used in)/from operating activities (420,327) (862,807) 442,480 Net cash flows from/(used in) investing activities 308,210 (461,184) 769,394 Net cash flows from/(used in) financing activities 1,336,727 843,757 492,970 Exchange gains/(losses) on cash and cash equivalents 23,494 (53,702) 77,196 Cash and cash equivalents at end of the period 2,048,844 800,740 1,248,104 Net Cash Used in Operating Activities Net cash outflow used in our operating activities decreased by $442 million to a net outflow of $420 million for the year ended December 31, 2023, compared to a net outflow of $863 million for the year ended December 31, 2022. The decrease in net cash outflow used in operating activities results primarily from an increase in net product sales related to VYVGART and VYVGART SC, partly offset by: i. the increase in research and development expenses incurred in relation to the manufacturing and clinical development activities of efgartigimod and the advancement of other clinical, preclinical and discovery-stage product candidate, ii. the increase in personnel expenses, marketing expenses and consulting expenses incurred for the commercial expansion of VYVGART and VYVGART SC, iii. the further increase in working capital as a result of our inventory levels, including prepaid inventory Net Cash Used in/from Investing Activities Investing activities for the year ended December 31, 2023, consist primarily of the net desinvestment of $272 million in current financial assets, and interests received, partly offset by payments related to regulatory and sales based milestones to Halozyme and investment in Oncoverity, resulting in a cash inflow of $308 million. Investing activities for the year ended December 31, 2022, consists primarily of net investment of $369 million in current financial assets, and purchase of a priority review voucher for $102 million, partly offset by interests received, resulting in a cash outflow of $461 million. 5.6.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Liquidity and Capital Resources | 261 |
| Net Cash Provided by Financing Activities Financing activities primarily consist of net proceeds from our private placements and public offerings of our securities and exercise of stock options. The net cash inflow from financing activities was $1,337 million for the year ended December 31, 2023, compared to a net cash inflow of $844 million for the year ended December 31, 2022. The net cash inflows were attributed to (i) $1.2 billion net cash proceeds from our global offering in July 2023, compared to $0.8 billion net cash proceeds from our global offering in February 2022 and (ii) $158 million proceeds received from the exercise of stock options in 2023, compared to $93 million for the year ended 2022. Operating and Capital Expenditure Requirements We have never achieved profitability and, as of December 31, 2023, we had accumulated losses of $2,405 million. Our losses resulted principally from costs incurred in research and development, preclinical testing and clinical development of our research programs, and from general and administrative costs associated with commercial roll out and expansion. We anticipate that our operating expenses will increase as we intend to continue to conduct research and development and continue our efforts to expand our sales, marketing and distribution infrastructure. Although we have generated net product sales of $1.2 billion from global product net sales of VYVGART and VYVGART SC for the treatment of gMG in fiscal year 2023, which supports our current path to profitability, we can provide no assurances that we will be able to achieve or sustain profitability based on this indication alone or that we will be able to receive regulatory approval of and commercialize VYVGART and VYVGART SC in other indications or in other countries. On the basis of current assumptions, we expect that our existing cash and cash equivalents and current financial assets will enable us to fund our operating expenses and capital expenditure requirements through at least the next twelve months. Our future equity capital will depend on multiple factors. Because of the numerous risks and uncertainties associated with the development and commercialization of efgartigimod and our other product candidates and discovery stage programs and because the extent to which we may enter into collaborations with third parties for the development of these product candidates is unknown, we are unable to estimate the amounts of increased capital outlays and operating expenses associated with completing the research and development of our product candidates. Our future capital requirements for efgartigimod and our other product candidates and discovery stage programs will depend on many factors, including: · the progress, timing and completion of preclinical testing and clinical trials for our current or any future product candidates; · the number of potential new product candidates we identify and decide to develop; · the time and costs involved in obtaining regulatory approval for our product candidates and any delays we may encounter as a result of evolving regulatory requirements or adverse results with respect to any of our product candidates; · selling and marketing activities undertaken in connection with the commercialization of VYVGART, VYVGART SC or potential commercialization of any of our current or any future product candidates, if approved, and costs involved in the creation of an effective sales and marketing organization; · manufacturing activities undertaken for VYVGART, VYVGART SC and potential commercialization of any of our current or any future product candidates, if approved, and costs involved in the creation of an effective supply chain; 5.6.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Liquidity and Capital Resources | 262 |
| ·將我們的組織發展到允許我們當前或任何未來候選產品的研究、開發和潛在商業化所需的規模所涉及的成本 ·提交專利申請以及維護和執行專利所涉及的成本,或者 針對第三方提出的索賠或侵權進行辯護的成本; ·維護我們現有的合作協議和簽訂新的合作協議 ; ·與全球經濟不確定性和政治不穩定有關的事態發展。 有關與我們未來資金需求相關的風險的更多信息,請參閲“風險 因素-與Argenx的財務狀況和額外資本需求有關的風險因素”。 財務和流動性政策 該公司已採取政策,將現金和現金等價物以及目前的金融資產與幾家信譽良好的銀行和金融機構進行投資。公司持有的現金和現金等價物主要與不同的銀行持有,這些銀行被 獨立評級,最低評級為‘A-’。本公司還以貨幣市場基金的形式持有短期投資基金,建議投資期限為6個月或更短,但歷史波動性較低。這些貨幣市場基金 是高流動性的投資,可以很容易地轉換為已知金額的現金。該公司採取了貨幣市場基金的平均評級必須為 “bbb”或更高的政策。 有關我們的國庫政策和流動性的更多信息,請參閲我們在截至2023年12月31日的年度報告中附上的合併財務報表中的附註26“金融 工具和金融風險管理”。 營運資本報表 根據歐盟委員會授權的條例(EU)2019/ 980附件11第3.1項,我們做出以下聲明: 我們認為,公司的營運資金足以滿足公司目前的 要求,至少從本年度報告之日起12個月內。 研發、專利和 許可證 有關我們研發政策的討論,請參閲《概覽》和《經營業績》。 5.6.4 5.6.5 5.7 ar Gr g oup enx Factors Risk Go Corporate vernance資本 分享財務 評審報表 財務非財務 信息 argenx 2023年年度報告研發、專利和許可證|263 |
| 趨勢信息 除本年度報告中其他地方披露的情況外,我們不瞭解本財政期間的任何趨勢、不確定性、需求、承諾或事件,這些趨勢、不確定性、需求、承諾或事件有可能對我們的淨收入、收入、盈利能力、流動性、資本資源或前景產生重大影響,或導致披露的財務信息不一定能反映未來的經營結果或財務狀況。 在FDA分別於2021年和2023年批准VYVGART和VYVGART SC在美國治療GMG後,我們從臨牀階段過渡到商業階段的生物技術公司。我們現在已經將VYVGART在美國、歐盟、日本、中國(通過我們的合作伙伴再鼎醫藥)、以色列(通過我們的合作伙伴麥迪遜)和加拿大商業化, VYVGART SC在美國和德國實現了商業化。我們正致力於在其他 司法管轄區擴大商業化,並推出新產品和候選產品,包括進入新的 適應症。 自2023年12月31日的資產負債表日期以來,集團的財務業績或財務狀況沒有重大變化。 有關更多信息,請參閲“概述”、“經營業績”、“流動資金和 資本資源”,並在“截至12月31日的年度的合併財務報表”中註明29項“承諾”。2023.“ 在提出的期間內,我們沒有,目前也沒有適用規則和條例所界定的任何資產負債表外安排,例如與未合併實體或金融合夥企業的關係,這些關係通常稱為 結構性融資或特殊目的實體,其目的是促進不需要反映在我們資產負債表上的融資交易。 合同義務 有關合同義務的討論,請參閲附註29。關於獨立審計師的信息 截至2023年12月31日的財政年度及截至2022年和2021年的財政年度的經審計的綜合財務報表已由我們的獨立審計師德勤會計師事務所(德勤)審計,並就這些財務報表出具了無保留的審計報告。簽署審計報告的德勤合夥人是荷蘭特許會計師協會(Koninklijke Nederlandse BeroepsOrganizatie van Account)的成員。德勤的辦公室位於荷蘭鹿特丹AP 3072 Wilhelminakade 1, 5.8 5.9 5.10 5.11 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 評審報表 財務非財務 信息 argenx 2023年年度報告趨勢信息|264 |
| Material Contracts and Related Party Transactions Material Contracts Our material contracts are described in sections “Collaborations and Licenses”, and “Distribution Agreements”. Related Party Transactions Since January 1, 2023, we have not entered into any transactions with any related parties which are – as a single transaction or in their entirety – material to us. In addition, since January 1, 2023, there has not been, nor is there currently proposed, any material transaction or series of similar material transactions to which we were or are a party in which any of the members of our Board of Directors or senior management, holders of more than 10% of any class of our voting securities, or any member of the immediate family of any of the foregoing persons, had or will have a direct or indirect material interest, other than the compensation and shareholding arrangements we describe in section “Share Classes and Principal Shareholders”, and the transactions we describe below. Transactions with Related Companies From time to time, in the ordinary course of our business, we may contract for services from companies in which certain of the members of our senior management or directors may serve as director or advisor. The costs of these services are negotiated on an at arm’s length basis and none of these arrangements are material to us. Agreements with Our Senior Management Other than as set forth in this Annual Report, there are no arrangements or understandings in place with major shareholders, customers, suppliers or others pursuant to which any member of our Board of Directors or senior management team has been appointed. We have entered into a management agreement with Tim Van Hauwermeiren as our CEO, our sole executive director. The key terms of his agreement are as follows: Tim Van Hauwermeiren Fixed-base compensation $655,787 Short-term variable compensation A target of 60% of the fixed-base compensation based on previously determined bonus targets established by the non-executive directors Pension contributions1) $22,821 Duration Indefinite 1) Amounts shown represent pension contributions paid during the year ended December 31, 2023. 5.12 5.12.1 5.12.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Contracts and Related Party Transactions | 265 |
| We may terminate Mr. Van Hauwermeiren’s services upon 18 months’ notice, or payment of 18 months’ pro-rated base compensation in lieu of notice. Mr. Van Hauwermeiren would be entitled to the same payment in lieu of notice in the event he terminates his services with us in circumstances in which it cannot reasonably be expected for him to continue providing services to us (and after our failure to remedy such conditions after being provided at least 14 days’ notice). Mr. Van Hauwermeiren would also be entitled to payment in lieu of notice in the event he terminated his services with us in certain cases of our failure to comply with obligations under applicable law or his agreement (and after our failure to remedy such non-compliance, if non-deliberate, after being provided at least 14 days’ notice). In these cases, there will be a full acceleration of the vesting of any outstanding stock options held by Mr. Van Hauwermeiren. There will be no notice period or payment in lieu of notice in certain cases of Mr. Van Hauwermeiren’s failure to comply with obligations under applicable law or his agreement. Mr. Van Hauwermeiren may be dismissed immediately as an executive director. Karl Gubitz, our chief financial officer, has an employment contract with our subsidiary, argenx US Inc., for an indefinite term. Keith Woods, our chief operating officer, had an employment contract with our subsidiary, argenx US Inc., for an indefinite term. His employment contract ended in March 2023. Karen Massey, our chief operating officer, joined argenx in March 2023 and has an employment contract with our subsidiary, argenx Switzerland SA, for an indefinite term. Peter Ulrichts, our chief scientific officer, since January 2023, has an employment contract with our subsidiary, argenx BV, for an indefinite term. Arjen Lemmen, our vice president corporate development and strategy, has an employment contract with our subsidiary, argenx BV, for an indefinite term. We may terminate his employment contract at any time, subject to a notice period and a severance payment of at least 12 months. Mr. Lemmen entered into a secondment agreement with argenx BV, under which Mr. Lemmen has been seconded from argenx BV to argenx US in the U.S. from August 1, 2022 until on or about July 31, 2024 (unless otherwise extended by the parties). In connection with his secondment, Mr. Lemmen receives a housing, schooling and cost of living allowance. Andria Wilk, our global head of quality, has an employment contract with our subsidiary, argenx BV, for an indefinite term. Malini Moorthy, our general counsel has an employment contract with our subsidiary, argenx US, for an indefinite term. Ms. Moorthy has also entered into a secondment agreement with argenx US, under which Ms. Moorthy has been seconded from argenx US to argenx BV and is based in Belgium for the period of April 1, 2023 through December 31, 2024 (unless otherwise extended by the parties). Luc Truyen, our head of research and development management operations and our chief medical officer, has an employment contract with our subsidiary, argenx US, for an indefinite term. Mr. Truyen entered into a secondment agreement with argenx US, under which Mr. Truyen has been seconded from argenx US to argenx BV and is based in Belgium for the period of April 1, 2022 through November 30, 2026 (unless otherwise extended by the parties). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Contracts and Related Party Transactions | 266 |
| Indemnification Agreements In connection with our initial U.S. public offering, we entered into indemnification agreements with each of our non-executive directors and each member of our senior management. We have entered into such agreements with each new non-executive director or member of our senior management when they have joined us since our initial U.S. public offering. Insofar as indemnification for liabilities arising under the Securities Act may be permitted to non-executive directors, officers or persons controlling us pursuant to the foregoing provisions, we have been informed that in the opinion of the SEC such indemnification is against public policy as expressed in the Securities Act and is therefore unenforceable. Related Party Transactions Policy In connection with our initial U.S. public offering, we entered into a related party transaction policy. Our Code of Conduct and our Board Rules also include specific rules of transactions with related parties. Property, plants and equipment We also lease office space in Amsterdam (the Netherlands), Boston (U.S.), Tokyo (Japan), Geneva (Switzerland), Munich (Germany), Issy Les Moulineaux (France), Vaughan Ontario (Canada), Gerrards Cross (UK) and Milan (Italy). In addition, our lease liabilities include a lease plan for company cars with maturity dates up to four years. For a discussion of contractual obligations, please see “Note 29 – Commitments” in our consolidated financial statements which are appended to our Annual Report for the period ended December 31, 2023. We have our principal executive, operational offices and laboratory space located in Zwijnaarde, Belgium. We have the following material facilities worldwide leased as of December 31, 2023, as set forth in the following table: Facility location Use Approx. size (m2 ) Lease expiry Zwijnaarde, Belgium (leased) Operations and Laboratory Space 4,678 September 30, 2028 Boston, Massachusetts (leased) Office Space 914 August 31, 2030 Tokyo, Japan (leased) Office Space 546 January 17, 2027 Environment, Health and Safety Our primary research and development activities take place in our facilities in Zwijnaarde, Belgium. For these activities we require, and have obtained, the necessary environmental and biohazard permits from the responsible governments, required by us for the manner in which we use said facilities. See section “Risk Factors”. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Contracts and Related Party Transactions | 267 |
| Employees As of December 31, 2023, we had 1,148 employees and 309 consultants, which we refer to as “contingent workers.” At each date shown below, we had the following number of employees, broken out by department and geography. At December 31, 2023 2022 2021 Function: Research and development 653 367 289 Selling, general and administrative 495 476 361 Total 1,148 843 650 Geography: Belgium 355 363 296 U.S. 454 340 276 Japan 116 75 57 The Netherlands 22 – – Switzerland 28 15 9 France 40 11 3 Germany 25 11 9 Canada 16 5 – UK 37 – – Italy 27 – – Spain 20 – – Other – remote 8 23 – Total 1,148 843 650 Collective bargaining agreements (CBAs) can be entered into in Belgium at the national, industry, or company levels. These CBAs are binding on both employers and employees. We have no trade union representation or CBAs at the company level, but we are subject to the national and chemical industry CBAs. The CBAs currently applicable to us relate to employment conditions such as wages, working time, job security, innovation and supplementary pensions. We have not had, and do not anticipate having, disputes on any of these subjects. CBAs may, however, change the employment conditions of our employees in the future and hence adversely affect our employment relationships. Insurance We maintain an insurance portfolio that is common and appropriate for our business. Our main insurances are commercial general liability insurances, including products liability insurance, director and officer liability insurance and our maritime insurance covering the risk of loss of product during transit and storage. 5.13 5.14 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Employees | 268 |
| Legal and Arbitration Proceedings From time to time we may become involved in legal, governmental or arbitration proceedings or be subject to claims arising in the ordinary course of our business. Regardless of the outcome, litigation can have an adverse impact on us because of defense and settlement costs, diversion of management resources and other factors. During the previous 12 months, there have not been any legal, governmental or arbitration proceedings (including any such proceedings which are pending or threatened of which we are aware) which may have, or have had in the recent past, significant effects on argenx and/or the Group’s financial position or profitability. Taxation This summary does not consider your particular circumstances. We urge you to consult your own independent tax advisors about the income, capital gains and/or transfer tax consequences to you in light of your particular circumstances of purchasing, holding and disposing of ordinary shares or ADSs. U.S. Federal Income Tax Considerations The following discussion is a summary under present law of certain material U.S. federal income tax considerations relating to the ownership and disposition of ADSs by a U.S. holder (as defined below). This summary addresses only the U.S. federal income tax considerations for U.S. holders that hold ADSs as capital assets (generally, property held for investment) and use the U.S. dollar as their functional currency. This summary does not address all U.S. federal income tax matters that may be relevant to a particular U.S. holder and is not a substitute for tax advice. This summary does not address tax considerations applicable to a holder of ADSs that may be subject to special tax rules including, without limitation, banks, financial institutions or insurance companies, brokers, dealers or traders in securities, currencies, commodities, or notional principal contracts, traders in securities that elect to mark-to-market, tax-exempt entities or organizations, including “individual retirement accounts” or “Roth IRAs”, real estate investment trusts, regulated investment companies, persons that hold the ADSs as part of a “hedging,” “integrated” or “conversion” transaction or as a position in a “straddle”, partnerships (including entities or arrangements classified as partnerships for U.S. federal income tax purposes) or other pass-through entities (including S-corporations), or persons that will hold the ADSs through such an entity, certain former citizens or long-term residents of the United States, persons that received the ADSs as compensation for the performance of services, persons subject to special tax accounting rules as a result of any item of gross income with respect to the shares being taken into account in an applicable financial statement, and holders that own directly, indirectly, or through attribution 10% or more of the voting power or value of our ordinary shares and ADSs. This summary does not address U.S. federal taxes other than the income tax (such as the Medicare surtax on net investment income, the estate, gift, or alternative minimum tax), any election to apply section 1400Z-2 of the U.S. Internal Revenue Code of 1986, as amended (the Code) to gains recognized with respect to ADSs, or any U.S. state, local, or non-U.S. tax considerations of the ownership and disposition of ADSs. For the purposes of this summary, a “U.S. holder” is a beneficial owner of ADSs that is (or is treated as), for U.S. federal income tax purposes, (i) an individual who is a citizen or resident of the United States, (ii) a corporation, or any other entity treated as a corporation for U.S. federal income tax purposes, created or organized in or under the laws of the United States, 5.15 5.16 5.16.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Legal and Arbitration Proceedings | 269 |
| any state thereof, or the District of Columbia, (iii) an estate, the income of which is subject to U.S. federal income taxation regardless of its source, or a trust, if a court within the United States is able to exercise primary supervision over its administration and one or more U.S. persons have the authority to control all of the substantial decisions of such trust. If a partnership (or other entity or arrangement treated as a partnership for U.S. federal income tax purposes) holds ADSs, the U.S. federal income tax consequences relating to an investment in those ADSs will depend in part upon the status of the partner and the activities of the partnership. A partnership that holds ADSs should consult its tax advisor regarding the U.S. federal income tax considerations for it and for its partners of owning and disposing of ADSs in its and their particular circumstances. In general, a U.S. holder that owns ADSs will be treated as the beneficial owner of the underlying shares represented by those ADSs for U.S. federal income tax purposes. Accordingly, no gain or loss will generally be recognized if a U.S. holder exchanges ADSs for the underlying shares represented by those ADSs. Persons considering an investment in the ADSs should consult their own taxad visors as to the particular tax consequences applicable to them relating to the ownership and disposition of ADSs, including the applicability of U.S. federal, state and local tax laws and non-U.S. tax laws. Distributions Although we do not currently plan to pay dividends, and subject to the discussion under “Passive Foreign Investment Company Considerations” below, the gross amount of distributions paid with respect to our ordinary shares including Dutch or Belgian tax withheld therefrom, if any (other than pro rata distribution), generally will be included in a U.S. holder’s gross income as foreign source ordinary dividend income when actually or constructively received to the extent such distribution is paid out of our current and accumulated earnings and profits as determined under U.S. federal income tax principles. Distributions in excess of our current and accumulated earnings and profits will be treated as a non-taxable return of capital and will be applied against and reduce, the U.S. holder’s adjusted tax basis in ADSs (but not below zero) and distributions in excess of earnings and profits and a U.S. holder’s adjusted tax basis will generally be taxable to the U.S. holder as either long-term or short-term capital gain depending upon whether the U.S. holder has held the ADSs for more than one year as of the time such distribution is received. However, since we do not calculate our earnings and profits under U.S. federal income tax principles, it is expected that any distribution will be reported as a dividend, even if that distribution would otherwise be treated as a non-taxable return of capital or as capital gain. Our dividends will not be eligible for the dividends-received deduction generally allowed to U.S. corporations. Dividends paid to non-corporate U.S. holders that satisfy a minimum holding period (during which they are not protected from the risk of loss) and certain other requirements may qualify for the preferential favorable tax rates applicable to qualified dividend income, provided that we are a “qualified foreign corporation” and we are not a PFIC as to the non-corporate U.S. holder in the taxable year of the dividend or the preceding taxable year. A qualified foreign corporation includes a non-U.S. corporation that is eligible for the benefits of a comprehensive income tax treaties with the United States. A non-U.S. corporation also will be considered to be a qualified foreign corporation with respect to any dividend it pays on shares which are readily tradable on an established securities market in the United States. Our ADSs are listed on the Nasdaq Global Select Market, which is an established securities market in the United States, and we expect our ADSs to be readily tradable on the Nasdaq Global Select Market. However, there can be no assurance that the ADSs will be considered readily tradable on an established securities market in the United States in any taxable year. U.S. holders should consult their own tax advisors regarding the application of these rules given their particular circumstances. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 270 |
| If dividends are subject to Dutch or Belgian withholding tax, a U.S. holder may be entitled, subject to generally applicable limitations, to claim a U.S. foreign tax credit for Dutch or Belgian withholding tax imposed at the appropriate rate. U.S. holders who do not elect to claim a credit for any foreign income taxes paid or accrued during the taxable year may instead claim a deduction of such taxes. The rules relating to the foreign tax credit are complex and recent changes to the foreign tax credit rules that apply to foreign taxes paid or accrued in taxable years beginning after December 27, 2021 introduced additional requirements and limitations. Each U.S. holder should consult its own tax advisors regarding the foreign tax credit rules. In general, the amount of a distribution paid to a U.S. holder in a foreign currency will be the dollar value of the foreign currency calculated by reference to the applicable exchange rate on the day the U.S. holder receives the distribution, regardless of whether the foreign currency is converted into USDs at that time. Any foreign currency gain or loss a U.S. holder realizes on a subsequent conversion of foreign currency into USDs will be U.S. source ordinary income or loss. If dividends received in a foreign currency are converted into USDs on the day they are received, a U.S. holder should not be required to recognize foreign currency gain or loss in respect of the dividend. Sale, Exchange or Other Taxable Disposition of ADSs Subject to the discussion under “-Passive Foreign Investment Company Considerations” below, a U.S. holder will generally recognize capital gain or loss on the sale, exchange or other taxable disposition of ADSs in an amount equal to the difference between the amount realized from such sale or exchange and the U.S. holder’s adjusted basis in the ADSs, each amount determined in USD. The adjusted tax basis in ADSs generally will be equal to the USD cost of such ADSs. Any such capital gain or loss generally will be long-term capital gain or loss if the U.S. holder’s holding period for such ADSs exceeds one year as of the date of sale or other disposition. Long-term capital realized by a non-corporate U.S. holder is generally eligible for a preferential reduced rates. The deductibility of capital losses for U.S. federal income tax purposes is subject to certain limitations. Any such gain or loss that a U.S. holder recognizes generally will be treated as U.S. source income or loss for foreign tax credit limitation purposes. Passive Foreign Investment Company Considerations In general, a non-U.S. corporation will be classified as a passive foreign investment company, or PFIC, for any taxable year in which, after applying certain look-through rules with respect to certain dividends, rents, interest or royalties received from its affiliates and taking into account its proportionate share of the income and assets of its 25% or more owned subsidiaries, either: (i) at least 75% of its gross income is “passive income” or (ii) at least 50% of the average quarterly value of its total gross assets is attributable to cash in excess of working capital requirements or assets that produce “passive income” or are held for the production of “passive income.” Passive income for this purpose generally includes dividends, interest, royalties, rents, gains from commodities and securities transactions, the excess of gains over losses from the disposition of assets which produce passive income. While we are treated as a publicly traded company for these purposes, the value of our assets, including goodwill and other intangibles, will be based on their fair market value, which will depend on the market value of our ordinary shares and ADSs, which are subject to change. Based on our historic and anticipated operations, the composition of our income and the projected composition and estimated fair market values of our assets, [我們不認為 我們在最近的納税年度是PFIC,]and do not expect to be classified as a PFIC for the current taxable year or for the foreseeable future. However, our possible status as a PFIC is a factual determination made annually after the close of each taxable year and, therefore, may be subject to change. Accordingly, there can be no assurance that we will not ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 271 |
| 在美國持有者持有美國存託憑證的任何一年內都是PFIC。本公司不打算 提供對其PFIC地位的任何年度評估。 如果我們在美國持有人擁有ADS的任何課税年度被歸類為PFIC,則 在出售或以其他方式處置(包括某些質押)該美國持有人的ADS時確認的收益將按比例分配給該美國持有人的持有期。分配給 銷售或處置的納税年度和我們成為PFIC之前的任何年度的金額將作為普通收入納税,分配給其他納税年度的金額將 按適用於該納税年度的個人或公司的最高税率徵税,並將對每個 該年度的由此產生的納税義務徵收利息費用。此外,如果美國持有者在 任何課税年度收到的美國存託憑證分派超過該持有者在前三個課税年度(或如果較短,則為美國持有者持有的美國存託憑證)期間收到的美國存託憑證年度分派平均值的125%,則該超出的分派將按相同方式徵税。 此外,不屬於超額分配的股息將不符合適用於個人和其他非法人獲得的合格股息收入的 優惠税率。 如果公司在您擁有美國存託憑證的任何納税年度是PFIC,則在您擁有ADS的 期間的後續所有年度中,本公司通常將繼續被視為您的PFIC,即使公司不再滿足 PFIC身份的門檻要求。某些選擇可能會導致股票的替代待遇(如按市值計價的待遇)。美國持有者應諮詢他們自己的税務顧問,以瞭解公司在任何課税年度可能的PFIC地位以及對他們的影響,包括這些選擇是否將 可用,如果是,在您的 特定情況下,替代處理的後果是什麼。 備份預扣和信息報告 美國持有者通常將遵守有關美國存託憑證和出售收益的信息報告要求,交換或處置在美國境內或通過與美國相關的金融中介機構支付的美國存託憑證 ,除非 美國持有者是一家公司或其他“豁免接受者”。此外,除非美國持有者提供正確的納税人識別碼和正式簽署的美國國税局W-9表格或以其他方式建立 豁免,否則美國持有者可能會受到此類付款的後備扣繳。備份預扣不是附加税,任何備份預扣的金額將被允許作為抵扣美國持有者的美國聯邦所得税責任的抵免 ,並可能使該持有者有權獲得退款,前提是所需信息及時提供給美國國税局。 外國資產報告 某些個人和某些由個人控制的實體的美國持有者可能被要求 報告與美國存託憑證權益有關的信息,受某些例外情況的限制 (包括由美國金融機構持有的股票的例外),方法是 將IRS Form 8938(指定外國金融資產報表)與其聯邦收入 納税申報單一起提交。未報告所需信息的投資者可能會受到重大處罰。敦促美國持有人就其擁有和處置ADSs. ar Gr g oup enx Factors Risk Go Corporate vernance資本的 信息報告義務(如果有)諮詢其税務顧問 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告|272 |
| 荷蘭重大税務後果 以下摘要概述了與收購、擁有和處置美國存託憑證有關的某些重大荷蘭税務後果。本摘要中提及的所有荷蘭和荷蘭法律分別僅指荷蘭王國的歐洲部分及其法律。摘要並不旨在提供可能與收購、擁有及處置美國存託憑證(ADS)相關的所有荷蘭税務方面的全面或 完整圖景,而根據適用法律,該持有者可能須 接受特別税務處理。本摘要基於荷蘭在本年度報告發布之日生效的税法和慣例,這些法律和慣例可能會發生變化,可能會對荷蘭的税收後果產生前瞻性或追溯性的影響。 本摘要不涉及荷蘭《2021年預扣税法》(Wet Bronasting 2021)所指的被視為附屬公司(Gelieerd)的美國存託憑證持有人的荷蘭税務後果。一般而言,美國存託憑證持有人如(I)在該公司擁有合資格權益,(Ii)該公司在該公司擁有合資格權益,或(Iii)第三方在該公司及該第三方均擁有合資格權益,則就該等目的而言,該持有人被視為該公司的附屬公司。政黨等同於其所屬的任何 政黨協作組。限定權益是指 允許持有者以能夠確定另一方的活動的方式對另一方的決定具有決定性影響的權益。在任何情況下,如果一方(直接或間接)擁有超過50%的 %,則被視為在另一方中擁有符合資格的權益。該另一方的投票權。 為繳納荷蘭所得税和公司所得税,股份或某些其他資產,包括與股份有關的存託憑證,可由受託人、基金會或類似實體或安排、“第三方”等第三方合法擁有,在某些 情況下,必須分配給(被視為)財產授予人、授予人或類似創始人、“財產授予人”,或在財產授予人死亡後,該財產授予人的受益人“受益人”,按照他們對此類信託或類似安排的財產授予人的財產的權利, 按“分離的私人資產”。 摘要不涉及作為個人且在公司擁有重大權益(Aanmerkelijk Belang)的美國存託憑證持有人的荷蘭税收後果。一般而言,如果美國存託憑證持有人單獨或與其配偶或合夥人和/或某些其他近親直接或間接持有,或作為分居私人資產的委託人或受益人持有,則美國存託憑證持有人將在公司中擁有重大權益 (I)(X)所有權,(Y)某些其他權利,如用益物權,或(Z)獲得 (無論是否已發行)的權利,佔公司總已發行和已發行資本(或任何類別的已發行和已發行資本)的5%或以上的股份(包括美國存託憑證),或(Ii)(X)與公司年度利潤的5%或以上或公司清算收益的5%或更多有關的利潤分享證書(Winstbewijzen)的所有權或(Y)某些其他權利,如用益物權。 此外,美國存託憑證持有人在公司中擁有重大權益,如果 無論是單獨還是與持有人的配偶或合夥人和/或某些其他近親一起,擁有公司發行的股份或利潤證書的股份或存託憑證的所有權或其他權利,而該股份或存託憑證佔相關總額的不到5%,且(A)符合上述 所述重大權益的一部分資格,且股份或與股份、利潤證書和/或權利有關的存託憑證已經或被視為已經部分處置,或(B)作為符合非確認收益待遇資格的交易的一部分被收購。 5.16.2 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年報|273 |
| Furthermore, this summary does not address the Dutch tax consequences of a holder of the ADSs who: · is an individual and receives income or realizes capital gains in respect of the ADSs in connection with such holder’s employment activities or in such holder’s capacity as (former) board member or (former) supervisory board member; · is a resident of any non-European part of the Netherlands; or · falls within the scope of the Dutch Minimum Taxation Act 2024 (Wet minimumbelasting 2024). Dividend Withholding Tax General The Company is generally required to withhold dividend withholding tax imposed by the Netherlands at a rate of 15% on dividends distributed by the company in respect of our ordinary shares underlying the ADSs. The expression “dividends distributed by the company” as used herein includes, but is not limited to: (a) distributions in cash or in kind, deemed and constructive distributions and repayments of paid-in capital (gestort kapitaal) not recognized for Dutch dividend withholding tax purposes; (b) liquidation proceeds, proceeds of redemption of our ordinary shares or, as a rule, consideration for the repurchase of our ordinary shares by the company in excess of the average paid-in capital recognized for Dutch dividend withholding tax purposes; (c) the par value of our ordinary shares issued to a holder of our ordinary shares or an increase of the par value of our ordinary shares, to the extent that it does not appear that a contribution, recognized for Dutch dividend withholding tax purposes, has been made or will be made; and (d) partial repayment of paid-in capital, recognized for Dutch dividend withholding tax purposes, if and to the extent that there are net profits (zuivere winst), unless (i) the shareholders at a General Meeting have resolved in advance to make such repayment and (ii) the par value of our ordinary shares concerned has been reduced by an equal amount by way of an amendment of our articles of association. Holders of the ADSs Resident in the Netherlands A holder of the ADSs that is an individual that is resident or deemed to be resident in the Netherlands for Dutch tax purposes is generally entitled, subject to the anti-dividend stripping rules described below, to a full credit against its income tax liability, or a full refund, of the Dutch dividend withholding tax. A holder of the ADSs that is a legal entity that is resident or deemed to be resident in the Netherlands for Dutch tax purposes is generally entitled, subject to the anti-dividend stripping rules described below, to a full credit against its corporate income tax liability of the Dutch dividend withholding tax. If and to the extent such legal entity cannot credit the full amount of Dutch dividend withholding tax in a given year, the Dutch dividend withholding tax may be carried forward and credited against its corporate income tax liability in subsequent years (without time limitation). A holder of the ADSs that is a legal entity that is resident or deemed to be resident in the Netherlands for Dutch tax purposes that is exempt from Dutch corporate income tax but that is not qualifying exempt investment institution (vrijgestelde beleggingsinstelling), is generally entitled, subject to the anti-dividend stripping rules described below, to an exemption at ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 274 |
| 來源(待完成必要的程序手續)或全額退還收到的股息的荷蘭股息預扣税。 如果美國存託憑證持有人可歸因於該非居民持有者在荷蘭的永久機構,且該持有者既不是荷蘭居民,也不被視為荷蘭居民,則一般情況也是如此。 居住在荷蘭境外的美國存託憑證持有人 為税務目的居住在荷蘭的國家的美國存託憑證持有人,可以,根據此類税收條約的條款和下文所述的反股息剝離規則,有資格就收到的股息獲得荷蘭股息預扣税的全部或部分豁免,或全部或部分退還。 美國存託憑證持有人,即(A)居住在(I)歐盟成員國、(Ii)冰島、 挪威或列支敦士登的法人實體,或(3)荷蘭已與其締結税收條約的國家(即非歐盟成員國、冰島、挪威或列支敦士登),(B)根據與第三國締結的税收條約的條款,在其居住國居住的國家不被視為居住在荷蘭尚未與其締結税收條約的國家(即非歐盟成員國、冰島、挪威或列支敦士登),但受反濫用規則和反股息剝奪規則的限制, 一般有權居住在荷蘭尚未與其締結税收條約的國家。如果公司持有公司至少5%的股份(股份或在某些情況下,投票權),或者如果持有低於5%的股份,且美國存託憑證的持有人是荷蘭居民,則可完全免除收到的股息的荷蘭預扣税,它將受益於參與豁免 (這可能包括另一關聯方在公司中持有5%或更多權益的情況)。 美國存託憑證持有人收到的股息,即法律實體(A)居住在(I)歐盟成員國、(Ii)冰島、挪威或 列支敦士登的税收,完全免除荷蘭預扣股息税。或(3)荷蘭與其締結了包括股息條款的税收條約的國家,在下列情況下不予批准:(X)持有該持有人的權益,以避免另一人的荷蘭股息預扣税為主要目的(S),並且(2)構成人為結構或一系列結構的一部分(例如,由於反映經濟現實的正當商業原因而不到位的結構), 或(Y)美國存託憑證持有人的職能類似於符合資格的投資機構(如下所述的財政)或合格的豁免投資機構(vrijsterelde 以下所述)。 美國存託憑證持有人,即居住在(I)歐盟成員國或(Ii)冰島、挪威或列支敦士登,或(Iii)與荷蘭交換税務信息的司法管轄區內的實體税務實體(即,第(Iii)項所述的持有人持有美國存託憑證作為有價證券投資(即這種持有不是為了在美國存託憑證持有人和公司之間建立或 維持持久和直接的經濟聯繫,並且不允許美國存託憑證持有人有效地參與公司的管理或控制)),它在其居住國是免税的,並且不具有與符合資格的投資機構(財政)或符合資格的免税投資機構(vrijsterelde )類似的職能,如果它 是荷蘭居民,則可以免徵荷蘭公司所得税根據下文所述的反股息剝離規則,一般有權在源頭獲得豁免(取決於完成必要的程序手續)或全額退還收到的股息的荷蘭預扣税 。免除全額退款總體上將使某些外國 養老基金、政府機構和某些政府控制的商業entities. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 argenx 2023年年報|275 |
| According to the anti-dividend stripping rules, no exemption, reduction, credit or refund of Dutch dividend withholding tax will be granted if the recipient of the dividend paid by the company is not considered the beneficial owner (uiteindelijk gerechtigde) of the dividend as defined in these rules. A recipient of a dividend is not considered the beneficial owner of the dividend if, as a consequence of a combination of transactions and tested at group level, (i) a person (other than the holder of the dividend coupon), directly or indirectly, partly or wholly benefits from the dividend, (ii) such person directly or indirectly retains or acquires a comparable interest in the ADSs, and (iii) such person is entitled to a less favorable exemption, refund or credit of dividend withholding tax than the recipient of the dividend distribution. The term “combination of transactions” includes transactions that have been entered into in the anonymity of a regulated stock market, the sole acquisition of one or more dividend coupons and the establishment of short-term rights or enjoyment on the ADSs (e.g., usufruct). The burden of proof to demonstrate that the recipient of a dividend qualifies as the beneficial owner of such dividend lies with the recipient, unless the amount of the withheld dividend withholding tax in respect of such recipient in the relevant calendar is €1,000 or less. Holders of the ADSs Resident in the U.S. Dividends distributed by the company to U.S. resident holders of the ADSs that are eligible for benefits under the Convention between the Netherlands and the U.S. for the avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes and Income, dated December 18, 1992 as amended by the protocol of March 8, 2004 (U.S. Tax Treaty), generally will be entitled to a reduced dividend withholding tax rate of 5% in case of certain U.S. corporate shareholders owning at least 10% of the company’s total voting power. Certain U.S. pension funds and tax-exempt organizations may qualify for a complete exemption from Dutch dividend withholding tax. Under the U.S. Tax Treaty such benefits are generally available to U.S. residents if such resident is the beneficial owner of the dividends, provided that such shareholder does not have an enterprise or an interest in an enterprise that is, in whole or in part, carried on through a permanent establishment or permanent representative in the Netherlands and to which enterprise or part of an enterprise the ADSs are attributable. A person may, however, not claim the benefits of the U.S. Tax Treaty if such person’s entitlement to such benefits is limited by the provisions of Article 26 (the limitation on benefits provision) of the U.S. Tax Treaty. The reduced dividend withholding tax rate can generally be applied at source upon the distribution of the dividends, provided that the proper forms have been filed in advance of the distribution. In the case of certain tax-exempt organizations, as a general rule, the so-called refund method applies; only when certain administrative conditions have been fulfilled may such tax-exempt organization use the exemption method. Irrespective of meeting the conditions of the relevant provisions of the U.S. Tax Treaty, dividends distributed by the company to a U.S. resident holder (i) who is a legal entity resident in the U.S. and (ii) that is in the U.S. under the terms of a tax treaty with a third state not considered to be resident for tax purposes in a country with which the Netherlands has not concluded a tax treaty that includes an article on dividends (not being a Member State of the European Union, Iceland, Norway or Liechtenstein), are generally, subject to the anti-dividend stripping rules described above, fully exempt from Dutch dividend withholding tax if the U.S. resident holder of ADSs holds an interest of at least 5% in the company or if it holds an interest of less than 5%, in either case where, had the holder of ADSs been a Dutch resident, it would have had the benefit of the participation exemption (this may include a situation where another related party holds an interest of 5% or more in the company). The full exemption from Dutch dividend withholding tax on dividends received by a U.S. holder of ADSs that is a legal entity is however not granted if (x) the interest held by such U.S. holder (i) is held with the avoidance of Dutch dividend withholding tax of another person as (one of) the main purpose(s) and (ii) forms part of an artificial structure or series of structures (such as ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 276 |
| structures which are not put into place for valid business reasons reflecting economic reality) or (y) the U.S. holder of ADSs has a similar function to a qualifying investment institution (fiscale beleggingsinstelling) or a qualifying exempt investment institution (vrijgestelde beleggingsinstelling). Taxes on Income and Capital Gains Holders of the ADSs Resident in the Netherlands: Individuals A holder of the ADSs, who is an individual resident or deemed to be resident in the Netherlands for Dutch tax purposes will be subject to regular Dutch income tax on the income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs by the holder thereof, if: (a) such holder of the ADSs has an enterprise or an interest in an enterprise, to which enterprise the ADSs are attributable; and/or (b) such income or capital gain forms “a benefit from miscellaneous activities” (resultaat uit overige werkzaamheden) which, for instance, would be the case if the activities with respect to the ADSs exceed “normal active asset management” (normaal, actief vermogensbeheer) or if income and gains are derived from the holding, whether directly or indirectly, of (a combination of) shares, debt claims or other rights (together, a “lucrative interest” (lucratief belang)) that the holder thereof has acquired under such circumstances that such income and gains are intended to be remuneration for work or services performed by such holder (or a related person), whether within or outside an employment relation, where such lucrative interest provides the holder thereof, economically speaking, with certain benefits that have a relation to the relevant work or services. If either of the abovementioned conditions (a) or (b) applies, income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs will in general be subject to Dutch income tax at the progressive rates up to 49.5%. If the abovementioned conditions (a) and (b) do not apply, a holder of the ADSs who is an individual, resident or deemed to be resident in the Netherlands for Dutch tax purposes will not be subject to taxes on income and capital gains in the Netherlands. Instead, such individual is generally taxed at a flat rate of 36% on deemed income from “savings and investments” (sparen en beleggen), which deemed income is determined on the basis of the amount included in the individual’s “yield basis” (rendementsgrondslag) at the beginning of the calendar year (minus a tax-free threshold; the yield basis minus such threshold being the tax basis). For the 2024 tax year, the deemed income derived from savings and investments will be a percentage of the tax basis up to 6.04% that is determined based on the actual allocation of (i) savings, (ii) other investments, and (iii) debts/liabilities within the individual’s yield basis. The tax-free threshold for 2024 is €57,000. The percentages to determine the deemed income will be reassessed every year. Holders of the ADSs Resident in the Netherlands: Corporate Entities A holder of the ADSs that is resident or deemed to be resident in the Netherlands for corporate income tax purposes, and that is: · a corporation; · another entity with a capital divided into shares; · a cooperative (association); or · another legal entity that has an enterprise or an interest in an enterprise to which the ADSs are attributable, ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 277 |
| 但不是: ·有資格的養老基金; ·有資格的投資機構(財政性投資機構)或有資格的豁免投資機構(Vrijsterelde Beleggingsinsting);或者 ·另一個免徵企業所得税的實體一般將被徵收常規的 荷蘭企業所得税,通常對來自美國存託憑證的收入和因收購、贖回和/或處置美國存託憑證而實現的收益徵收25.8%(19%的利潤,包括200,000歐元)的税率,除非 參與豁免(Deelnemingsrijering)適用。在荷蘭境外居住的美國存託憑證持有人:個人 不在荷蘭居住或被視為在荷蘭居住的人,將不需要就來自美國存託憑證的收入和因收購、贖回和/或處置美國存託憑證而獲得的收益繳納任何荷蘭税(上述荷蘭股息預扣税除外),除非: (A)該持有人擁有一家企業或在該企業中擁有全部或部分權益,而該企業或企業的部分或全部是通過在荷蘭的常設機構(Aste Inrichting)或永久 代表(Aste Verteve Genwodiger)經營的,視情況而定,美國存託憑證是可歸因的;或 (B)這種收入或資本收益形成“從荷蘭的其他活動中獲得的利益”(結果是荷蘭的其他活動),例如,如果在荷蘭與美國存託憑證有關的活動超過“正常的活躍資產管理”(Normaal,Actief mogensbeheer),或者如果這種收入和收益直接或間接地來自持有(組合)股票、債務、債權或其他權利(合在一起,持有者在這樣的情況下獲得的“有利可圖的利益”(Lucatief Belang),該收入和收益旨在 該持有者(或相關人士)在荷蘭從事的工作或服務的全部或部分報酬,無論是在僱傭關係內還是在僱傭關係之外,如果從經濟上講,這種有利可圖的利益為其持有人提供了與相關工作或服務有關的 利益。 如果上述(A)或(B)條件之一適用,公司分派股息或因收購、贖回和/或出售美國存託憑證而實現的任何收益的收入或資本利得一般將按最高49.5%的累進税率繳納荷蘭所得税。 居住在荷蘭境外的美國存託憑證持有人:法律實體和其他實體 美國存託憑證持有人,即法人實體,資本分為股份的另一實體, 協會、基金會或基金或信託,非荷蘭居民或因公司所得税而被視為在荷蘭居住的人,將不需要就來自美國存託憑證的收入和因收購、贖回和/或處置美國存託憑證而實現的收益繳納任何荷蘭税(上述荷蘭股息預扣税除外),除非: ·該持有者擁有一家企業或在該企業中擁有全部或部分權益, 通過荷蘭的常設機構(Vaste Inrichting)或永久代表(Vaste Vertegenwoordiger)進行的,且美國存託憑證歸屬於哪個企業或企業的一部分,視具體情況而定;Or ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年年報税務|278 |
| · such holder has a substantial interest (aanmerkelijk belang) in the company, that (i) is held with the avoidance of Dutch income tax of another person as (one of) the main purpose(s) and (ii) forms part of an artificial structure or series of structures (such as structures which are not put into place for valid business reasons reflecting economic reality). If one of the abovementioned conditions applies, income derived from the ADSs and the gains realized upon the acquisition, redemption and/or disposal of the ADSs will, in general, be subject to Dutch regular corporate income tax, levied at a rate of 25.8% (19% over profits up to and including €200,000), unless, and to the extent that, with respect to a holder as described under (a), the participation exemption (deelnemingsvrijstelling) applies. Gift, Estate and Inheritance Taxes Holders of the ADSs Resident in the Netherlands Gift tax may be due in the Netherlands with respect to an acquisition of the ADSs by way of a gift by a holder of the ADSs who is resident or deemed to be resident of the Netherlands at the time of the gift. Inheritance tax may be due in the Netherlands with respect to an acquisition or deemed acquisition of the ADSs by way of an inheritance or bequest on the death of a holder of the ADSs who is resident or deemed to be resident of the Netherlands, or in case of a gift by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while that individual, at the time of the individual’s death, is resident or deemed to be resident in the Netherlands. For purposes of Dutch gift and inheritance tax, an individual with the Dutch nationality will be deemed to be resident in the Netherlands if such individual has been resident in the Netherlands at any time during the 10 years preceding the date of the gift or such individual’s death. For purposes of Dutch gift tax, an individual not holding the Dutch nationality will be deemed to be resident of the Netherlands if such individual has been resident in the Netherlands at any time during the 12 months preceding the date of the gift. Holders of the ADSs Resident Outside the Netherlands No gift, estate or inheritance taxes will arise in the Netherlands with respect to an acquisition of the ADSs by way of a gift by, or on the death of, a holder of the ADSs who is neither resident nor deemed to be resident of the Netherlands, unless, in the case of a gift of the ADSs by an individual who at the date of the gift was neither resident nor deemed to be resident in the Netherlands, such individual dies within 180 days after the date of the gift, while being resident or deemed to be resident in the Netherlands. Certain Special Situations For purposes of Dutch gift, estate and inheritance tax, (i) a gift by a third party will be construed as a gift by the settlor, and (ii) upon the death of the settlor, as a rule such settlor’s beneficiaries will be deemed to have inherited directly from the settlor. Subsequently, such beneficiaries will be deemed the settlor, grantor or similar originator of the separated private assets for purposes of the Dutch gift, estate and inheritance tax in case of subsequent gifts or inheritances. For the purposes of the Dutch gift and inheritance tax, a gift that is made under a condition precedent is deemed to have been made at the moment such condition precedent is satisfied. If the condition precedent is fulfilled after the death of the donor, the gift is deemed to be made upon the death of the donor. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 279 |
| Value Added Tax No Dutch value added tax will arise in respect of or in connection with the subscription, issue, placement, allotment or delivery of the ADSs. Other Taxes and Duties No Dutch registration tax, capital tax, custom duty, transfer tax, stamp duty or any other similar documentary tax or duty, other than court fees, will be payable in the Netherlands in respect of or in connection with the subscription, issue, placement, allotment or delivery of the ADSs. Residency A holder of the ADSs will not be treated as a resident, or a deemed resident, of the Netherlands for tax purposes by reason only of the acquisition, or the holding, of the ADSs or the performance by the company under the ADSs. Material Belgian Tax Consequences The paragraphs below present a summary of certain Belgian federal income tax consequences of the ownership and disposal of ADSs by an investor that purchases such ADSs. The summary is based on laws, treaties and regulatory interpretations in effect in Belgium on the date of this Annual Report, all of which are subject to change, including changes that could have retroactive effect. Investors should appreciate that, as a result of evolutions in law or practice, the eventual tax consequences may be different from what is stated below. The tax legislation of the investor’s country of residence may have an impact on the income received from the ADSs. This summary does not purport to address all tax consequences of investments in, the ownership and disposal of ADSs, and does not take into account the specific circumstances of particular investors, some of which may be subject to special rules, or the tax laws of any country other than Belgium. This summary does not describe the tax treatment of investors that are subject to special rules, such as banks, insurance companies, collective investment undertakings, dealers in securities or currencies, persons that hold, or will hold, ADSs as a position in a straddle, share-repurchase transaction, conversion transactions, synthetic security or other integrated financial transactions. This summary does not address the tax regime applicable to ADSs held by Belgian tax residents through a fixed base or a permanent establishment (PE) situated outside Belgium. This summary does not address the local taxes that may be due in connection with an investment in shares, other than the local surcharges which generally vary between 0% and 9% of the investor’s income tax liability in Belgium. In addition to the assumptions mentioned above, it is also assumed in this discussion that for purposes of the domestic Belgian tax legislation, the owners of ADSs will be treated as the owners of the ordinary shares represented by such ADSs. However, this assumption has not been confirmed by or verified with the Belgian Tax Authorities. Investors should consult their own advisors regarding the tax consequences of an investment in the ADSs in light of their particular situation, including the effect of any state, local or other national laws, treaties and regulatory interpretations thereof. 5.16.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 280 |
| For the purposes of this summary, a resident investor is: · an individual subject to Belgian personal income tax (personenbelasting/impôt des personnes physiques), i.e. (i) an individual having its domicile in Belgium, (ii) when not having its domicile in Belgium, an individual having its seat of wealth in Belgium, or (iii) an individual assimilated to a resident for purposes of Belgian tax law; · a company (as defined by Belgian tax law) subject to Belgian corporate income tax (vennootschapsbelasting/impôt des sociétés), i.e., a corporate entity having its principal establishment, administrative seat or effective place of management in Belgium (and that is not excluded from the scope of the Belgian corporate income tax). A company having its registered seat in Belgium shall be presumed, unless the contrary is proved, to have its principal establishment, administrative seat or effective place of management in Belgium; or · a legal entity subject to the Belgian tax on legal entities (rechtspersonenbelasting/impôt des personnes morales), i.e., a legal entity other than a company subject to Belgian corporate income tax having its principal establishment, administrative seat or effective place of management in Belgium. A non-resident investor is any individual, company or legal entity that does not fall in any of the three previous classes. Dividends For Belgian income tax purposes, the gross amount of all benefits paid on or attributed to the ADSs is generally treated as a dividend distribution. By way of exception, the repayment of capital carried out in accordance with applicable Dutch company law provisions is not treated as a dividend distribution to the extent that such repayment is imputed on fiscal capital. This fiscal capital includes, in principle, the actual paid-up statutory share capital and, subject to certain conditions, the paid-up share premiums and the cash amounts subscribed to at the time of the issue of profit-sharing certificates. However, a repayment of capital is not fully imputed on fiscal capital if the company also has certain reserves. Indeed, in such case, a reimbursement of capital is proratedly imputed on, on the one hand, fiscal capital and, on the other hand, taxed reserves (whether or not incorporated in capital) and tax-exempt reserves incorporated in capital (according to a specific priority rule). The part imputed on the reserves is treated as a dividend distribution subject to applicable tax rules. Belgian withholding tax of 30% is normally levied on dividends by any intermediary established in Belgium that is in any way involved in the processing of the payment of non-Belgian sourced dividends (e.g., a Belgian financial institution). This withholding tax rate is subject to such relief as may be available under applicable domestic or tax treaty provisions. The Belgian withholding tax is calculated on the dividend amount after deduction of any non-Belgian dividend withholding tax. In the case of a redemption of the ADSs, the redemption distribution (after deduction of the part of the fiscal capital represented by the redeemed ADSs) will be treated as a dividend subject to a Belgian withholding tax of 30%, subject to such relief as may be available under applicable domestic or tax treaty provisions. No withholding tax will be triggered if this redemption is carried out on a stock exchange and meets certain conditions. In the event of our liquidation, any amounts distributed in excess of the fiscal capital will in principle be subject to the 30% withholding tax, subject to such relief as may be available under applicable domestic or tax treaty provisions. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 281 |
| Under Belgian law, non-Belgian dividend withholding tax is not creditable against Belgian income tax and is not reimbursable to the extent that it exceeds Belgian income tax. Please refer to Item E. “Taxation – Material Dutch Tax Consequences – Dividend Withholding Tax” for a description of withholding tax that may be imposed on dividends by the Netherlands. Belgian Resident Individuals For Belgian resident individuals who acquire and hold ADSs as a private investment, the Belgian dividend withholding tax fully discharges their personal income tax liability. They may nevertheless need to report the dividends in their personal income tax return if no intermediary established in Belgium was in any way involved in the processing of the payment of the non-Belgian sourced dividends. Moreover, even if an intermediary established in Belgium was involved, they can opt to report the income in their personal income tax return. If (and only if) the dividends are reported, they will normally be eligible for a tax exemption with respect to ordinary dividends in an amount of up to €833 (for income year 2024) per year and per taxpayer (Article 21, first subsection, 14°, of the Belgian Income Tax Code (ITC)). For the avoidance of doubt, all reported dividends (not only dividends distributed on our ADSs) are taken into account to assess whether the said maximum amount is reached. The abovementioned exempted amount is not applicable to redemption and liquidation dividends. Where the beneficiary needs or, as applicable, opts to report them, dividends will normally be taxable at the lower of the generally applicable 30% Belgian withholding tax rate on dividends or, in case globalization is more advantageous, at the progressive personal income tax rates applicable to the taxpayer’s overall declared income. In addition, if the dividends are reported, the Belgian dividend withholding tax levied at source may be credited against the personal income tax due and is reimbursable to the extent that it exceeds the personal income tax due, provided that the dividend distribution does not result in a reduction in value of or a capital loss on our ADSs. The latter condition is not applicable if the individual can demonstrate that it has held ADSs in full legal ownership for an uninterrupted period of 12 months prior to the payment or attribution of the dividends. For Belgian resident individual investors who acquire and hold the ADSs for professional purposes, the Belgian withholding tax does not fully discharge their Belgian income tax liability. Dividends received must be reported by the investor and will, in such a case, be taxable at the investor’s personal income tax rate increased with local surcharges. Belgian withholding tax levied may be credited against the personal income tax due and is reimbursable to the extent that it exceeds the income tax due, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution may not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable if the investor can demonstrate that it has held the full legal ownership of the ADSs for an uninterrupted period of 12 months prior to the payment or attribution of the dividends. Belgian Resident Companies Dividends received by Belgian resident companies are exempt from Belgian withholding tax provided that the investor satisfies the identification requirements in Article 117, §11 of the Royal Decree implementing the ITC. For Belgian resident companies, the gross dividend income (after deduction of any non-Belgian withholding tax but including any Belgian withholding tax) must be declared in the corporate income tax return and will be subject to a corporate income tax rate of 25%, except that a reduced corporate income tax rate of 20% applies to small companies and medium sized enterprises (as defined by Article 1:24, §1 to §6 of the Belgian Code on Companies and Associations) on the first €100,000 of taxable profits (subject to certain conditions). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 282 |
| Belgian resident companies can generally (although subject to certain limitations) deduct 100% of the gross dividend received from their taxable income (Dividend Received Deduction) provided that at the time of a dividend payment or attribution: (i) the Belgian resident company holds ADSs representing at least 10% of our share capital or a participation with an acquisition value of at least €2,500,000 (it being understood that only one out of the two tests must be satisfied); (ii) the shares representing our share capital have been or will be held in full ownership for an uninterrupted period of at least one year; and (iii) the conditions described in Article 203 of the ITC (relating to the taxation of the underlying distributed income and the absence of abuse), or the Article 203 of the ITC Taxation Condition, are met (Conditions for Dividend Received Deduction). Conditions (i) and (ii) above are, in principle, not applicable for dividends received by an investment company within the meaning of Article 2, §1, 5°, f) ITC. The Conditions for the application of the Dividend Received Deduction Regime depend on a factual analysis and for this reason the availability of this regime should be verified upon each dividend distribution. Any Belgian dividend withholding tax levied at source can be credited against the ordinary Belgian corporate income tax and is reimbursable to the extent it exceeds such corporate income tax, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution does not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable: (i) if the taxpayer can demonstrate that it has held the ADSs in full legal ownership for an uninterrupted period of 12 months immediately prior to the payment or attribution of the dividends or (ii) if, during that period, the ADSs never belonged to a taxpayer other than a Belgian resident company or a non-resident company that has, in an uninterrupted manner, invested the ADSs in a PE in Belgium. Belgian resident Organizations for Financing Pensions For organizations for financing pensions (OFPs) i.e., Belgian pension funds incorporated under the form of an OFP (organisme voor de financiering van pensioenen/organisme de financement de pensions) within the meaning of Article 8 of the Belgian Law of October 27, 2006, dividend income generally does not constitute taxable income. Dividends distributed through the intervention of a Belgian intermediary are generally subject to Belgian dividend withholding tax. If dividends are paid or attributed without the intervention of a Belgian intermediary, the applicable Belgian withholding tax will have to be reported and paid by the OFP to the Belgian tax administration. The Belgian dividend withholding tax can in principle be credited against the OFPs’ corporate income tax and is reimbursable to the extent it exceeds the corporate income tax due. However, such Belgian withholding cannot be credited by an OFP if the shares on which the dividends are paid have not been held uninterruptedly in full ownership for at least 60 days, unless the OFP demonstrates that the dividends are not connected to an arrangement (or a series of arrangements) that is not genuine (kunstmatig/pas authentique) and has been put in place for the main purpose or one of the main purposes of obtaining this withholding tax credit. Other Belgian resident Taxable Legal Entities For taxpayers subject to the Belgian income tax on legal entities, the Belgian dividend withholding tax in principle fully discharges their income tax liability. If the dividend is paid outside Belgium without the intervention of a Belgian paying agent and without the deduction of Belgian withholding tax, the legal entity is in principle required to declare and pay the 30% withholding tax to the Belgian tax authorities. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 283 |
| Belgian Non-Resident Individuals and Companies Dividend payments on the ADSs through a professional intermediary in Belgium will, in principle, be subject to the 30% withholding tax, unless the shareholder is resident in a country with which Belgium has concluded a double taxation agreement and delivers the requested affidavit. Non-resident investors can also obtain an exemption of Belgian dividend withholding tax if they are the owners or usufructors of the ADSs and they deliver an affidavit confirming that they have not allocated the ADSs to business activities in Belgium and that they are non-residents, provided that the dividend is paid through a Belgian credit institution, stock market company or recognized clearing or settlement institution. If the ADSs are acquired by a non-resident investor in connection with a business in Belgium, the investor must report any dividends received, which are taxable at the applicable non-resident individual or corporate income tax rate, as appropriate. Any Belgian withholding tax levied at source can be credited against the non-resident individual or corporate income tax and is reimbursable to the extent it exceeds the income tax due, subject to two conditions: (i) the taxpayer must own the ADSs in full legal ownership on the dividend record date and (ii) the dividend distribution does not result in a reduction in value of or a capital loss on the ADSs. The latter condition is not applicable if (i) the non-resident individual or the non-resident company can demonstrate that the ADSs were held in full legal ownership for an uninterrupted period of 12 months immediately prior to the payment or attribution of the dividends or (ii) with regard to non-resident companies only, if, during the said period, the ADSs have not belonged to a taxpayer other than a resident company or a non-resident company which has, in an uninterrupted manner, invested the ADSs in a Belgian PE. Non-resident companies that have invested the ADSs in a Belgian establishment can deduct up to 100% of the gross dividends included in their taxable profits if, at the date dividends are paid or attributed, the Conditions for Dividend Received Deduction are satisfied. Application of the Dividend Received Deduction depends, however, on a factual analysis to be made upon each distribution and its availability should be verified upon each distribution. Capital Gains and Losses on ADSs Belgian Resident Individuals In principle, Belgian resident individuals acquiring the ADSs as a private investment should not be subject to Belgian capital gains tax on the disposal of the ADSs; capital losses are not tax deductible. Capital gains realized in a private (i.e., non-professional) context on the transfer for consideration of shares by a private individual, are taxable at 33% (plus local surcharges) if the capital gain is deemed to be realized outside the scope of the normal management of the individual’s private estate. Capital losses are, however, not tax deductible in such event. Capital gains realized in a private (i.e., non-professional) context on the transfer for consideration of shares of a Belgian company to a foreign company with its fiscal residency outside the EEA, by a private individual, who held alone or jointly with his/her family, directly or indirectly, more than 25% of the shares of that Belgian company, are taxable at a flat rate of 16.5% (plus local surcharges). Gains realized by Belgian resident individuals upon the redemption of the ADSs or upon our liquidation are generally taxable as a dividend. Belgian resident individuals who hold the ADSs for professional purposes are taxable at the ordinary progressive personal income tax rates (plus local surcharges) on any capital gains realized upon the disposal of the ADSs, except for ADSs held for more than five years, which are taxable at a flat rate of 16.5% (plus local surcharges). Capital losses on the ADSs incurred ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 284 |
| by Belgian resident individuals who hold the ADSs for professional purposes are in principle tax deductible. Belgian Resident Companies Belgian resident companies are normally not subject to Belgian capital gains taxation on gains realized upon the disposal of our ADSs provided that (i) the shares represent at least 10% of our share capital or a participation with an acquisition value of at least €2,500,000 (it being understood that only one out of the two tests must be satisfied), (ii) the Article 203 ITC Taxation Condition is satisfied and (iii) the ADSs have been held in full legal ownership for an uninterrupted period of at least one year immediately preceding the disposal. If one of the above conditions is not met, the capital gains realized upon the disposal of our ADSs by a Belgian resident company are taxable at the ordinary corporate income tax rate of, currently, 25%, unless the reduced corporate income tax rate of 20% on the first €100,000 of taxable profits applies (see above). Capital losses on our ADSs incurred by resident companies are as a general rule not tax deductible. Our ADSs held in the trading portfolios (handelsportefeuille/portefeuille commercial) of qualifying credit institutions, investment enterprises and management companies of collective investment undertakings which are subject to the Royal Decree of 23 September 1992 on the annual accounts of credit institutions, investment firms and management companies of collective investment undertakings (Koninklijk besluit van 23 september 1992 op de jaarrekening van de kredietinstellingen, de beleggingsondernemingen en de beheervennootschappen van instellingen voor collectieve belegging/arrêté royal du 23 septembre 1992 relatif aux comptes annuels des établissements de crédit, des entreprises d’investissement et des sociétés de gestion d’organismes de placement collectif) are subject to a different regime. The capital gains on such shares are taxable at the ordinary corporate income tax rate of 25%. Capital losses on such shares are tax deductible. Internal transfers to and from the trading portfolio are assimilated to a realization. Capital gains realized by Belgian resident companies (both ordinary Belgian resident companies and qualifying credit institutions, investment enterprises and management companies of collective investment undertakings) upon the redemption of our ADSs or upon our liquidation are, in principle, subject to the same taxation regime as dividends. See Item E. “Taxation – Dividends.” Belgian resident OFPs OFPs are, in principle, not subject to Belgian capital gains taxation realized upon the disposal of the ADSs, and capital losses are not tax deductible. Capital gains realized by Belgian OFPs upon the redemption of ADSs or upon our liquidation will in principle be taxed as dividends. Other Belgian Taxable Legal Entities Belgian resident legal entities subject to the legal entities income tax are, in principle, not subject to Belgian capital gains taxation on the disposal of ADSs. Capital gains realized by Belgian resident legal entities upon the redemption of ADSs or upon our liquidation will in principle be taxed as dividends. Capital losses on ADSs incurred by Belgian resident legal entities are not tax deductible. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 285 |
| Belgian Non-Resident Individuals and Companies Non-resident individuals or companies are, in principle, not subject to Belgian income tax on capital gains realized upon disposal of the ADSs, unless such ADSs are held as part of a business conducted in Belgium through a Belgian establishment. In such a case, the same principles apply as described with regard to Belgian individuals (holding the shares for professional purposes) or Belgian companies. Non-resident individuals who do not use the shares for professional purposes and who have their fiscal residence in a country with which Belgium has not concluded a tax treaty or with which Belgium has concluded a tax treaty that confers the authority to tax capital gains on the ADSs to Belgium, might be subject to tax in Belgium if the capital gains are obtained or received in Belgium and arise from transactions which are to be considered speculative or beyond the normal management of one’s private estate. See Item E. “Taxation – Capital Gains and Losses on ADSs – Belgian Resident Individuals.” Such non-resident individuals might therefore be obliged to file a tax return and should consult their own tax advisor. Capital gains realized by non-resident individuals or non-resident companies upon the redemption of ADSs or upon our liquidation will, in principle, be subject to the same taxation regime as dividends. Tax on Stock Exchange Transactions Upon the issue of the ADSs (primary market), no Tax on Stock Exchange Transactions (taks op beursverrichtingen/taxe sur opérations de bourse) is due. The purchase and the sale and any other acquisition or transfer for consideration of ADSs (secondary market transactions) is subject to the Tax on Stock Exchange Transactions if (i) it is executed in Belgium through a professional intermediary, or (ii) deemed to be executed in Belgium, which is the case if the order is directly or indirectly made to a professional intermediary established outside of Belgium, either by private individuals with habitual residence in Belgium, or legal entities for the account of their seat or establishment in Belgium (both, a Belgian Investor). The Tax on Stock Exchange Transactions is levied at a rate of 0.35% of the purchase price, capped at €1,600 per transaction and per party. A separate tax is due by each party to the transaction, and both taxes are collected by the professional intermediary. However, if the intermediary is established outside of Belgium, the tax will in principle be due by the Belgian Investor, unless that Belgian Investor can demonstrate that the tax has already been paid. Professional intermediaries established outside of Belgium can, subject to certain conditions and formalities, appoint a Belgian Stock Exchange Tax Representative, which will be liable for the Tax on Stock Exchange Transactions in respect of the transactions executed through the professional intermediary. If the Stock Exchange Tax Representative would have paid the Tax on Stock Exchange Transactions due, the Belgian Investor will, as per the above, no longer be the debtor of the Tax on Stock Exchange Transactions. No Tax on Stock Exchange Transactions is due on transactions entered into by the following parties, provided they are acting for their own account: (i) professional intermediaries described in Article 2, 9° and 10° of the Belgian Law of August 2, 2002; (ii) insurance companies described in Article 2, §1 of the Belgian Law of July 9, 1975; (iii) professional retirement institutions referred to in Article 2,1° of the Belgian Law of October 27, 2006 concerning the supervision on institutions for occupational pension; (iv) collective investment institutions; (v) regulated real estate companies; and (vi) Belgian non-residents provided they deliver a certificate to their financial intermediary in Belgium confirming their non-resident status. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 286 |
| The EU Commission adopted on February 14, 2013 the Draft Directive on a Financial Transaction Tax (FTT). The Draft Directive currently stipulates that once the FTT enters into force, the Participating Member States shall not maintain or introduce taxes on financial transactions other than the FTT (or VAT as provided in the Council Directive 2006/112/EC of November 28, 2006 on the common system of value added tax). For Belgium, the Tax on Stock Exchange Transactions should thus be abolished once the FTT enters into force. Due to the lack of progress in the negotiations on the Draft Directive, the European Commission has announced that it would present a proposal for a new own resource based on the FTT by June 2024 (with a view to its introduction by January 1, 2026). Annual Tax on Securities Accounts A Law of 17 February 2021 introduced a new Belgian Annual Tax on Securities Accounts, which entered into effect on February 26, 2021. The Annual Tax on Securities Accounts is a subscription tax, levied on securities accounts and not on the holders thereof. A securities account is defined as an account on which financial instruments can be credited and debited. The tax applies to securities accounts held both in Belgium and abroad when the account holder is a Belgian resident or when the account forms part of the assets of a Belgian establishment of a non-Belgian resident. The tax applies to natural persons residing in Belgium, as well as to companies and legal entities (subject to the tax for legal entities) that are established in Belgium. The tax is also applicable to securities accounts held by non-Belgian residents (both natural persons and legal persons) if the securities account is held in Belgium. If the applicable double tax treaty however allocates the right to tax capital to the jurisdiction of residence, Belgium would be prevented from applying the Annual Tax on Securities Accounts to the Belgian securities accounts held by non-Belgian residents. As described above, the tax applies whether or not the account is held in Belgium if the account forms part of the assets of a Belgian establishment of a non-Belgian resident. The Annual Tax on Securities Accounts is applicable to securities accounts of which the average value of the assets amounts to more than €1,000,000 during the reference period. In principle, this reference period starts on 1 October and ends on 30 September of the following year. The aforementioned threshold is assessed on the average value of the assets in the securities account at reference points within the reference period (in principle December 31st, March 31st, June 30th and September 30th). The threshold is assessed per securities account and not per account holder. The applicable tax rate is 0.15%, which is levied on the average value of the assets held in the securities account that exceeds the €1,000,000 threshold. It is however limited to 10% of the difference between the average value and the threshold of €1,000,000, in order to avoid that the Annual Tax on Securities Accounts would result in reducing the value of the securities account below the €1,000,000 threshold. The Annual Tax is in principle withheld, reported and paid by the Belgian intermediary. If the intermediary is established outside of Belgium, the tax must in principle be reported and paid by the account holder, unless the account holder can demonstrate that the tax has already been reported and paid by an intermediary. Intermediaries established outside of Belgium can, subject to certain conditions and formalities, appoint a Belgian Annual Tax on Securities Accounts Representative, which will be liable for reporting and paying the Annual Tax on Securities Accounts in respect of securities accounts in scope of the Annual Tax that are held through such intermediaries. If the Annual Tax on Securities Accounts Representative would have paid the Annual Tax on Securities Accounts due, the account holder will, as per the above, no longer be the debtor of the Annual Tax on Securities Accounts. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 287 |
| 然而,證券賬户的年度税收不適用於 相關法律所列的活躍在金融或基金行業的某些類別的賬户持有人所持有的證券賬户(如信貸機構、保險公司、投資公司和 某些集體投資企業)。但是,如果不符合資格的第三方對證券賬户的價值有直接或間接的債權要求,則這些豁免不適用。 強烈建議潛在投資者就以下問題尋求自己的專業建議: 證券賬户年度税對其個人税務的可能影響 民事責任的強制執行 我們是根據荷蘭法律註冊成立的歐洲上市公司(Societas Europaea或SE) 。我們的幾乎所有資產都位於美國境外。因此,投資者可能無法在美國境內向此類人員送達訴訟程序 或在美國法院對他們或我們執行,包括 根據美國聯邦證券法的民事責任條款作出的判決。 美國和荷蘭目前沒有條約規定在民事和商業事務中相互承認和執行判決(仲裁裁決除外)。因此,由美國法院作出的最終付款判決,無論是否完全基於美國證券法, 都不會自動在荷蘭得到承認或強制執行。為了獲得可在荷蘭強制執行的判決,被美國法院作出最終和決定性判決的一方將被要求向荷蘭有管轄權的法院提出索賠。該方當事人可向荷蘭法院提交美國法院作出的最終判決。本法院在評估相關美國法院作出的判決時將有一定程度的自由裁量權。根據荷蘭最高法院的判例法,荷蘭法院原則上必須使該法院關於合同義務的最終和可執行的判決具有決定性效力,而不對所裁決的實體事項進行復審或重新訴訟,前提是:(I)所涉美國法院根據國際公認的理由接受管轄權以接受管轄權,(Ii)在該法院進行的訴訟符合正當程序原則(應按正當程序進行分拆),(Iii)該判決不違反荷蘭的公共政策,以及(Iv)該判決與荷蘭法院在同一當事人之間作出的判決或外國法院在同一事項爭議中基於相同訴訟理由作出的先前判決不相牴觸,只要該先前判決滿足使其在荷蘭具有約束力所需的條件。荷蘭法院可能會 拒絕承認和執行懲罰性賠償或其他不符合荷蘭法律秩序的裁決。此外,荷蘭法院可以減少美國法院給予的損害賠償金額,並僅在補償實際損失或損害賠償所必需的範圍內才承認損害賠償。美國法院在荷蘭的判決的執行和承認僅受《荷蘭民事訴訟法典》的規定管轄。 執行美國法院判決的原始訴訟或與美國聯邦或州證券法的民事責任條款有關的訴訟不能在比利時直接執行 。美國和比利時目前沒有條約規定在民事和商事事務中相互承認和執行判決,但仲裁裁決除外。因此,由美國法院作出的最終付款判決,無論是否完全基於美國證券法,都不會自動在比利時得到承認或強制執行。為了使美國法院基於民事責任支付款項的最終判決在比利時領土上產生任何影響,因此 5.16.4 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度税務報告|288 |
| required that this judgment be recognized and be declared enforceable by a Belgian court pursuant to the relevant provisions of the PIL Code. Recognition or enforcement does not imply a review of the merits of the case and is irrespective of any reciprocity requirement. A U.S. judgment will, however, not be recognized or declared enforceable in Belgium if it infringes upon one or more of the grounds for refusal which are exhaustively listed in article 25 of the PIL Code. In addition to recognition or enforcement, a judgment by a federal or state court in the U.S. against us may also serve as evidence in a similar action in a Belgian court if it meets the conditions required for the authenticity of judgments according to the law of the state where it was rendered. In addition, with regard to enforcements by legal proceedings in Belgium (including the recognition of foreign court decisions in Belgium), a registration tax at the rate of 3% of the amount of the judgment is payable by the debtor, if the sum of money which the debtor is ordered to pay by a Belgian court, or by a foreign court judgment that is either (i) automatically enforceable and registered in Belgium, or (ii) rendered enforceable by a Belgian court, exceeds €12,500. The registration tax is payable by the debtor. The debtor is liable for the payment of the registration tax, in the proportion determined by the decision ordering payment or liquidation or determining priority for creditors made or established against it. The debtor(s) are jointly and severally liable in the event that they are ordered to pay jointly and severally. A stamp duty is payable as of the second certified copy of an enforcement judgment rendered by a Belgian court, with a maximum of €1,450. Dutch and Belgian civil procedure differ substantially from U.S. civil procedure in a number of respects. Insofar as the production of evidence is concerned, U.S. law and the laws of several other jurisdictions based on common law provide for pre-trial discovery, a process by which parties to the proceedings may prior to trial compel the production of documents by adverse or third parties and the deposition of witnesses. Evidence obtained in this manner may be decisive in the outcome of any proceeding. No such pre-trial discovery process exists under Dutch or Belgian law. Subject to the foregoing and service of process in accordance with applicable treaties, investors may be able to enforce in the Netherlands or Belgium judgments in civil and commercial matters obtained from U.S. federal or state courts. However, no assurance can be given that those judgments will be enforceable. In addition, it is doubtful whether a Dutch or Belgian court would accept jurisdiction and impose civil liability in an original action commenced in the Netherlands or Belgium and predicated solely upon U.S. federal securities laws. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Taxation | 289 |
| 財務報表 6.1合併財務報表291 6.2合併財務報表附註299 6.3 argenx SE截至2023年12月31日止年度的公司財務報表344 6 argenx 2023年年度報告財務報表|290 |
| 6 Financial Statements Consolidated Financial Statements Consolidated Statements of Financial Position As of December 31, (in thousands of $) Note 2023 2022 2021 Assets Non‑current assets Property, plant and equipment 4 22,675 16,234 15,844 Intangible assets 5 125,228 174,901 171,684 Deferred tax asset 24 97,211 79,222 32,191 Research and development incentive receivables 76,706 47,488 32,707 Investment in joint venture 27, 1 9,912 1,323 – Prepaid expenses 7 47,327 – – Other non-current assets 6 39,662 40,894 54,876 Total non‑current assets 418,721 360,064 307,303 Current assets Inventories 7 310,550 228,353 109,076 Prepaid expenses 8 134,072 76,022 58,946 Trade and other receivables 9 496,687 275,697 38,221 Research and development incentive receivables 2,584 1,578 – Financial assets 10 1,131,000 1,391,808 1,002,052 Cash and cash equivalents 11 2,048,844 800,740 1,334,676 Total current assets 4,123,737 2,774,197 2,542,971 Total assets 4,542,458 3,134,261 2,850,274 6.1 6.1.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Financial Statements | 291 |
| As of December 31, (in thousands of $) Note 2023 2022 2021 Equity and liabilities Equity 12 Equity attributable to owners of the parent Share capital 7,058 6,640 6,233 Share premium 5,651,497 4,309,880 3,462,775 Translation differences 131,543 129,280 131,684 Accumulated losses (2,404,844) (2,109,791) (1,400,197) Other reserves 712,253 477,691 333,729 Total equity 4,097,507 2,813,699 2,534,224 Non-current liabilities Provisions for employee benefits 1,449 870 417 Lease liabilities 22 15,354 9,009 7,956 Deferred tax liabilities 24 5,155 8,406 6,438 Total non-current liabilities 21,958 18,285 14,811 Current liabilities Lease liabilities 22 4,646 3,417 3,509 Trade and other payables 14 414,013 295,679 293,415 Tax liabilities 4,334 3,181 4,315 Total current liabilities 422,993 302,277 301,239 Total liabilities 444,951 320,562 316,050 Total equity and liabilities 4,542,458 3,134,261 2,850,274 The accompanying notes form an integral part of these consolidated financial statements. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Financial Position | 292 |
| Consolidated Statements of Profit or Loss Year Ended December 31, (in thousands of $ except for shares and EPS) Note 2023 2022 2021 Product net sales 15, 18 1,190,783 400,720 – Collaboration revenue 16 35,533 10,026 497,277 Other operating income 17 42,278 34,520 42,141 Total operating income 1,268,594 445,267 539,418 Cost of sales (117,835) (29,431) – Research and development expenses 19 (859,492) (663,366) (580,520) Selling, general and administrative expenses 20 (711,905) (472,132) (307,644) Loss from investment in joint venture (4,411) (677) – Total operating expenses (1,693,643) (1,165,607) (888,164) Operating loss (425,049) (720,341) (348,746) Financial income 23 107,386 27,665 3,633 Financial expense 23 (906) (3,906) (4,578) Exchange gains/(losses) 23 14,073 (32,732) (50,053) Loss for the year before taxes (304,496) (729,314) (399,743) Income tax benefit/(expense) 24 9,443 19,720 (8,522) Loss for the year (295,053) (709,594) (408,265) Loss for the year attributable to: Owners of the parent (295,053) (709,594) (408,265) Weighted average number of shares outstanding 57,169,253 54,381,371 51,075,827 Basic and diluted (loss) per share (in $) 25 (5.16) (13.05) (7.99) The accompanying notes form an integral part of these consolidated financial statements. 6.1.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Profit or Loss | 293 |
| Consolidated Statements of Comprehensive Income (Loss) Year Ended December 31, (in thousands of $) Note 2023 2022 2021 Loss for the year (295,053) (709,594) (408,265) Items that may be reclassified subsequently to profit or loss, net of tax Currency translation differences, arisen from translating foreign activities 2,263 (2,404) (3,048) Items that will not be reclassified subsequently to profit or loss, net of tax Fair value gain/(loss) on investments in equity instruments designated as at FVTOCI 6 (1,915) (18,267) (39,290) Other comprehensive income/(loss), net of income 348 (20,671) (42,338) Total comprehensive loss attributable to: Owners of the parent (294,705) (730,266) (450,603) The accompanying notes form an integral part of these consolidated financial statements. 6.1.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Comprehensive Income (Loss) | 294 |
| Consolidated Statements of Cash Flows Year Ended December 31, (in thousands of $) Note 2023 2022 2021 Operating loss (425,049) (720,341) (348,746) Adjustments for non-cash items Amortization of intangible assets 5 105,674 99,766 776 Depreciation of property, plant and equipment 4 5,633 4,576 5,091 Provisions for employee benefits 573 459 260 Expense recognized in respect of share-based payments 13 232,974 157,026 179,366 Fair value gains on financial assets at fair value through profit or loss 6 – (4,256) (11,152) Non-cash revenue – – (75,000) Loss from investment in joint venture 27, 1 4,411 677 – Other non-cash expenses 2,074 – – (73,710) (462,093) (249,405) Movements in current assets/liabilities (Increase)/decrease in trade and other receivables 9 (185,694) (222,260) (31,632) (Increase)/decrease in inventories 7 (83,030) (119,277) (83,880) (Increase)/decrease in other current assets (59,024) (18,294) (30,990) Increase/(decrease) in trade and other payables 14 95,600 329 134,892 Increase/(decrease) in deferred revenue – current – – (46,327) Movements in non-current assets/liabilities (Increase)/decrease in other non‑current assets 6 (29,416) (16,220) (13,975) (Increase)/decrease in non-current prepaid expense 7 (47,327) – – Increase/(decrease) in deferred revenue – non-current – – (269,039) Net cash flows used in operating activities, before interest and taxes (382,601) (837,815) (590,356) Interest paid (211) (851) (684) Income taxes paid (37,515) (24,141) (15,772) 6.1.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Cash Flows | 295 |
| Year Ended December 31, (in thousands of $) Note 2023 2022 2021 Net cash flows used in operating activities (420,327) (862,807) (606,812) Purchase of intangible assets 5 (43,000) (102,986) (117,811) Purchase of property, plant and equipment 4 (812) (837) (3,623) (Increase)/decrease in current financial assets 10 – – (228,239) Purchase of current financial investments 10 (1,271,730) (1,694,046) – Sale of current financial investments 10 1,543,999 1,325,540 – Interest received 92,753 13,146 2,603 Investment in joint venture (13,000) (2,000) – Net cash flows from/(used in) investing activities 308,210 (461,184) (347,070) Principal elements of lease payments 22 (3,801) (4,165) (3,855) Proceeds from issue of new shares, gross amount 12 1,196,731 760,953 1,091,326 Issue costs paid 12 (821) (781) (528) Exchange (losses)/gains from currency conversion on proceeds from issue of new shares (1,507) 410 966 Payment of employee withholding taxes relating to restricted stock unit awards (12,138) (5,855) – Proceeds from exercise of stock options 12 158,263 93,195 33,433 Net cash flows from financing activities 1,336,727 843,757 1,121,342 Increase/(decrease) in cash and cash equivalents 1,224,610 (480,234) 167,460 Cash and cash equivalents at the beginning of the period 800,740 1,334,676 1,216,803 Exchange gains/(losses) on cash and cash equivalents 23,494 (53,702) (49,587) Cash and cash equivalents at the end of the period 2,048,844 800,740 1,334,676 The accompanying notes form an integral part of these consolidated financial statements. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Cash Flows | 296 |
| Consolidated Statements of Changes in Equity (in thousands of $) Attributable to owners of the parent Share capital Share premium Accumulated losses Translation differences Share-based payment and income tax deduction on share-based payments Fair value movement on investment in equity instruments designated as at FVTOCI Total equity attributable to owners of the parent Total equity Balance at January 1, 2021 5,744 2,339,033 (991,932) 134,732 186,474 – 1,674,051 1,674,051 Loss for the year (408,265) (408,265) (408,265) Other comprehensive income/(loss) (3,048) (39,290) (42,338) (42,338) Total comprehensive income/(loss) for the year (408,265) (3,048) (39,290) (450,603) (450,603) Income tax benefit from excess tax deductions related to share-based payments 7,179 7,179 7,179 Share-based payment 179,366 179,366 179,366 Issue of share capital 430 1,090,896 1,091,326 1,091,326 Transaction costs for equity issue (528) (528) (528) Exercise of stock options 59 33,374 33,433 33,433 Balance year ended December 31, 2021 6,233 3,462,775 (1,400,197) 131,684 373,019 (39,290) 2,534,224 2,534,224 Loss for the year (709,594) (709,594) (709,594) Other comprehensive income/(loss) (2,404) (18,267) (20,671) (20,671) Total comprehensive income/(loss) for the year (709,594) (2,404) (18,267) (730,266) (730,266) Income tax benefit from excess tax deductions related to share-based payments 3,946 3,946 3,946 Share-based payment 158,282 158,282 158,282 6.1.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Changes in Equity | 297 |
| (in thousands of $) Attributable to owners of the parent Share capital Share premium Accumulated losses Translation differences Share-based payment and income tax deduction on share-based payments Fair value movement on investment in equity instruments designated as at FVTOCI Total equity attributable to owners of the parent Total equity Issue of share capital 294 760,659 760,953 760,953 Transaction costs for equity issue (781) (781) (781) Exercise of stock options 113 93,082 93,195 93,195 Ordinary shares withheld for payment of employees’ withholding tax liability (5,855) (5,855) (5,855) Balance year ended December 31, 2022 6,640 4,309,880 (2,109,791) 129,280 535,247 (57,557) 2,813,699 2,813,699 Loss for the year (295,053) (295,053) (295,053) Other comprehensive income/(loss) 2,263 (1,915) 348 348 Total comprehensive income/(loss) for the year (295,053) 2,263 (1,915) (294,705) (294,705) Income tax benefit from excess tax deductions related to share-based payments 2,310 2,310 2,310 Share-based payment 234,168 234,168 234,168 Issue of share capital 288 1,196,444 1,196,732 1,196,732 Transaction costs for equity issue (821) (821) (821) Exercise of stock options 130 158,133 158,263 158,263 Ordinary shares withheld for payment of employees’ withholding tax liability (12,139) (12,139) (12,139) Balance year ended December 31, 2023 7,058 5,651,497 (2,404,844) 131,543 771,725 (59,472) 4,097,507 4,097,507 Please refer to note 12 for more information on the share capital and movement in number of shares. See also note 13 for more information on the share-based payments. The accompanying notes form an integral part of these consolidated financial statements. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Consolidated Statements of Changes in Equity | 298 |
| Notes to the Consolidated Financial Statements 1. General Information about the Company argenx SE is a Dutch European public company with limited liability incorporated under the laws of the Netherlands. The company (COC 24435214) has its official seat in Rotterdam, the Netherlands, and its registered office is at Laarderhoogtweg 25, 1101 EB Amsterdam, the Netherlands. An overview of the company and its subsidiaries (the Company) are described in note 31. argenx SE is a publicly traded company with ordinary shares listed on Euronext Brussels under the symbol “ARGX” since July 2014 and with American Depositary Shares listed on Nasdaq under the symbol “ARGX” since May 2017. 2. Material Accounting Policy Information The Company’s material accounting policies are summarized below. 2.1 Statement of compliance and basis of preparation The consolidated financial statements are prepared in accordance with the International Financial Reporting Standards and the interpretations issued by the IASB’s International Financial Reporting Interpretation Committee as adopted by the European Union (EU-IFRS) and in accordance with the legal requirements of Part 9 of Book 2 of the Dutch Civil Code. The consolidated financial statements provide a general overview of the Company’s activities and the results achieved. They present fairly the entity’s financial position, its financial performance and cash flows, on a going concern basis. The material accounting policy information applied in the preparation of the above consolidated financial statements are set out below. All amounts are presented in thousands of dollar, unless otherwise indicated, rounded to the nearest $ ‘000. The consolidated financial statements have been approved for issue by the Company’s Board of Directors (the “Board”) on March 19, 2024. 2.2 Adoption of new and revised standards New standards and interpretations applicable for the annual period beginning on January 1, 2023 · Amendments to IAS 1 – Presentation of Financial Statements and IFRS Practice Statement 2 – Making Materiality Judgements. The amendments to IAS 1 require companies to disclose their material accounting policy information rather than their significant accounting policies. The amendments to IFRS Practice Statement 2 provide guidance on how to apply the concept of materiality to accounting policy disclosures. As result the Company revised its accounting policy disclosure in the consolidated financial statements and removed accounting policy information that the Company deemed to relate to immaterial transactions or other events or conditions. 6.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Consolidated Financial Statements | 299 |
| No other standards and interpretations for the annual period beginning on January 1, 2023 have any material impact on the consolidated financial statements. New standards and interpretations issued, but not yet applicable for the annual period beginning on January 1, 2023 · Amendments to IAS 12 – issued International Tax Reform – Pillar Two Model Rules. On 23 May 2023, the International Accounting Standards Board (the IASB or Board) issued International Tax Reform – Pillar Two Model Rules – Amendments to IAS 12 which clarified the application of IAS 12 income taxes arising from tax law enacted or substantively enacted to implement the OECD/G20 Inclusive Framework on Base Erosion and Profit Shifting Pillar Two model rules. Based on current information, management expects that the Company could become subject to the Pillar Two Directive and implementing domestic laws as early as 2025. Thus, there is no impact for argenx in 2023. The company is currently in the process of determining the impact, if any, for 2025. Based on the preliminary analysis, we do not expect the Pillar Two Rules to have a material impact on our effective tax rate. It is unclear if the Pillar Two model rules create additional temporary differences, whether to remeasure deferred taxes for the Pillar Two model rules, and which tax rate to use to measure deferred taxes. In response to this unclarity, the amendments mentioned above introduced a mandatory temporary exception to the requirements of IAS 12 under which a company does not recognize or disclose information about deferred tax assets and liabilities related to the Pillar Two model rules. We applied the temporary exception in financial year 2023. We have not early adopted any other standard, interpretation, or amendment that has been issued but is not yet effective. Of the standards that are not yet effective, we expect no standard to have a material impact on the financial statements in the period of initial application. 2.3 Basis of consolidation The consolidated financial statements include the financial statements of the Company and entities controlled by the Company (its subsidiaries). Control is achieved when the Company: · has power over the investee; · is exposed, or has rights, to variable returns from its involvement with the investee; and · has the ability to use its power to affect its returns. The Company reassesses whether or not it controls an investee if facts and circumstances indicate that there are changes to one or more of the three elements of control listed above. The results of the subsidiaries are included in the consolidated statements of profit or loss and consolidated statements of other comprehensive income (loss) from the effective date of acquisition up to the date when control ceases to exist. When necessary, adjustments are made to the financial statements of subsidiaries to bring their accounting policies into line with those used by other members of the Group. All intercompany transactions and unrealized gains on transactions between group companies are eliminated. Unrealized losses are also eliminated unless the transaction provides evidence of an impairment of the transferred asset. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Accounting Policy Information | 300 |
| 2.4 Foreign currency transactions 2.4.1 Functional and presentation currency Items included in the consolidated financial statements of each of the entities are valued using the currency of their economic environment in which the entity operates. The consolidated financial statements are presented in USD ($), which is the Company’s presentation currency. 2.4.2 Transactions and balances Transactions in foreign currencies are translated at the exchange rate ruling at the date of the transaction. Monetary assets and liabilities denominated in foreign currencies are translated at the exchange rate ruling at the reporting date. Foreign exchange differences arising on translation are recognized in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss) as “Exchange gains/(losses)”. Non‑monetary assets and liabilities denominated in foreign currencies are translated at the foreign exchange rate ruling at the date of the transaction. 2.4.3 Financial statements of foreign entities For foreign entities using a different functional currency than USD: · assets and liabilities for each balance sheet presented are translated at the closing rate at the date of the balance sheet. · income and expenses for each statement presenting profit or loss and statements of other comprehensive income (loss) are translated at average exchange rates (unless this average is not a reasonable approximation of the cumulative effect of the rates prevailing on the transaction dates, in which case income and expenses are translated at the rate on the dates of the transactions). · all resulting exchange differences are recognized in the statements of other comprehensive income (loss). 2.5 Intangible assets 2.5.1 Internally generated intangible assets Expenditure on research activities is recognized as an expense in the period in which it is incurred. Due to uncertainties inherent to the development and registration with the relevant healthcare authorities of its products, the Company estimates that the conditions for capitalization per IAS 38 can not be met before the regulatory procedures required by such healthcare authorities have been finalized. Also once regulatory approval has been obtained, an internally generated intangible asset arising from development is capitalized if, and only if, all of the criteria under IAS 38 have been demonstrated. 2.5.2 Acquired In-Process R&D and Acquired R&D available for use Upfront payments and development milestone payments for “Acquired In-Process R&D obtained through in-licensing arrangements are capitalized as intangible assets under “Acquired In-Process R&D” upon meeting the IAS 38 capitalization criteria. These intangibles are considered as intangible assets with definite useful lives and are carried at cost less accumulated impairment losses. “Acquired In-Process R&D” is not amortized, but is evaluated for potential impairment on an annual basis or when facts and circumstances warrant. Any impairment charge is recorded in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss) under “Research and development expense”. Once an asset included in “Acquired In-Process R&D” has received marketing approval from a regulatory authority, it is recorded under “Acquired R&D available for use” category. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Accounting Policy Information | 301 |
| 通過許可內安排獲得的監管里程碑付款和基於銷售的里程碑研發付款在滿足國際會計準則38資本化標準後,將被資本化為無形資產,歸入“已獲得的可供使用的研發”項下。所有被歸類為“可供使用的已獲得研發”項下的無形資產均被視為使用年限有限的無形資產,並按減去累計攤銷和累計減值損失後的成本計提。當 公司根據資產的事實和情況確定指示時,對“收購的可供使用的研發”進行潛在減值評估。任何減值費用均記入綜合損益表和綜合其他全面收益(虧損)表中的“銷售成本”項下。“收購研發 可供使用”在“銷售成本”項下以直線方式攤銷估計的使用壽命,即收購研發的當前專利保護壽命和組合產品的專利保護壽命中較長的一個。 2.5.3。其他無形資產 其他無形資產可能包括優先審查憑證(“PRV”),公司 可以使用該憑證獲得FDA對其未來提交的監管文件的優先審查,或者 可能出售或轉讓給第三方。PRV最初按成本計量,當事件或情況表明賬面價值可能無法收回時,每年對減值進行審查。任何減值費用均記入 “研發費用”項下的合併損益表和綜合其他全面收益(虧損)表。使用在 綜合損益表和其他 綜合損益(虧損)表中記錄的PRV結果攤銷,以及隨後的無形資產註銷。 2.6應收研發獎勵 本期和非本期研發獎勵應收款項與在比利時的研究和開發獎勵費用產生的 退款有關,並計入綜合損益表和其他綜合收益表(虧損)在“其他 營業收入”項下,當相關支出已經發生並且有合理的 保證將獲得研發獎勵時。 2.7存貨 存貨按成本或可變現淨值入賬,以最低者為準。成本包括 採購成本、轉換成本以及將庫存運至其當前位置和狀況所產生的其他成本。如果執行銷售的預期銷售價格減去完成成本(可變現淨值)低於賬面金額,則確認賬面金額超出其可變現淨值的金額進行減記。 庫存中包括的產品除商業活動外,還可用於 臨牀前和臨牀計劃,以及免費、體恤和預先批准的訪問計劃。當這些產品專門用於此渠道時,將分別計入“研發費用”或 “銷售、一般和管理費用”。 在監管部門批准這些產品之前,我們會將與這些產品相關的庫存成本資本化,或者在生產設施中生產的庫存極有可能銷售時,我們會將這些產品的庫存成本資本化。資本化的決定是基於與正在考慮的產品或生產設施的預期監管批准相關的特定事實和情況。對產品是否被認為極有可能可銷售進行評估,包括,但不限於,特定產品或設施在審批過程中取得了多大進展。任何已知的安全性或有效性問題和其他impediments. ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審查報表 財務非財務 信息 Argenx 2023年年度報告材料會計政策信息|302 |
| Previously capitalized costs related to pre-launch inventories could be required to be written down upon a change in such judgement or due to a denial or delay of approval by regulatory bodies, a delay in commercialization or other potential factors, which will be recorded under “Research and development expenses” in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss). 2.8 Trade and other receivables Trade and other receivables are designated as financial assets measured at amortized cost. They are initially measured either at their invoiced amounts or at transaction price, in the absence of a significant financing component less adjustments for estimated revenue deductions such as rebates, chargebacks and returns. All receivables are subsequently measured at amortized cost, which generally corresponds to nominal value less expected credit loss provision. Loss allowance for expected credit losses are established using a simplified approach of forward-looking expected credit loss model (ECL), which includes possible default events on the trade receivables over the entire holding period of the trade receivable. These provisions represent the difference between the trade receivable’s carrying amount in the consolidated statements of financial position and the estimated collectible amount. Charges for loss allowance for expected credit losses are recorded under “Selling, general and administrative expenses” in the consolidated statements of profit or loss and consolidated statements of other comprehensive income (loss). 2.9 Current financial assets Current financial assets measured at amortized costs comprise of term accounts that have an initial maturity equal or less than 12 months, but exceeding 3 months. Current financial assets measured at fair value through profit or loss comprise of money market funds. Interests on Current financial assets is reported under Cash Flow from investment activities under “Interest received”. 2.10 Cash and cash equivalents Cash are financial assets measured at amortized cost and comprise of cash at bank. Cash equivalents measured at amortized cost comprise of term accounts that have an initial maturity of less than 3 months that are subject to an insignificant risk of changes in values. Those are used by the Company in the management of short-term commitments. Cash and cash equivalents exclude restricted cash, which is presented in the consolidated statements of financial position under the line “Other non-current assets”. Cash equivalents measured at fair value through profit or loss comprise of money market funds that are readily convertible to cash and are subject to insignificant risk of changes in value. These financial assets are used by the Company in the management of the short-term commitments. Interests on Cash equivalents is reported under Cash Flow from investment activities under “Interest received”. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Accounting Policy Information | 303 |
| 2.11貿易及其他應付款項 貿易及其他應付款項由自資產負債表日期起計不足一年的負債組成,一般不計息,並於財政年度內持續結算 。它們還包括與公司研發活動有關的應計費用、毛淨應計項目和短期員工福利。 貿易和其他應付款最初按其交易價格計量,在初始確認後 按攤餘成本計量。 短期員工福利包括支付給公司員工的工資和獎金的應付款和應計項目。它們被確認為員工提供相應服務的 期間的費用。 2.12租賃 公司在合同開始時評估合同是否為租賃或包含租賃。 公司確認與其為承租人的所有租賃安排有關的使用權資產和相應的租賃負債,但短期租賃(定義為租賃期限為12個月或更短的租賃)和低價值資產租賃除外。就該等租賃而言,本公司按直線法將租賃付款確認為租賃期內的營運開支,除非另一個系統基準更能代表租賃資產的經濟利益的耗用時間 模式。 租賃負債最初按開始日期未支付的租賃付款的現值計量,並使用租賃中隱含的利率貼現。如果這一利率 不能輕易確定,承租人使用其遞增借款利率。租賃負債隨後通過增加賬面金額以反映租賃負債的利息(使用實際利息法)和通過減少賬面金額以反映所支付的租賃付款來計量。租賃負債在 綜合財務狀況報表中單獨列示。 使用權資產包括相應租賃負債的初始計量、在開始日期或之前支付的租賃付款、減去收到的任何租賃獎勵 以及任何初始直接成本。它們隨後按成本減去累計折舊和減值損失計量。使用權資產按標的資產較短的租賃期和使用年限進行折舊。如果租賃轉讓了標的資產的所有權,或者使用權資產的成本反映了公司希望 行使購買選擇權,則相關的使用權資產將在標的資產的使用年限內折舊。使用權資產於財務狀況綜合報表“物業、廠房及設備”列示。 2.13金融工具初步按公允價值或交易價格確認,而 其後根據本公司管理金融資產的模式及金融資產的合約現金流特徵,按國際財務報告準則第9號按攤餘成本或公允價值計量。當預期從該工具流出的現金流在一年內到期時,金融資產被歸類為流動資產。 AgomAb Treeutics NV的利潤份額:公司持有非流動金融資產的投資,該投資基於IFRS 9,通過 損益按公允價值指定為金融資產。上市投資的公允價值以該證券在每個報告日的收盤價為基礎。由於沒有活躍的權益工具市場, 公司採用估值技術來確定公允價值。Fair ar Gr g oup enx Factors Risk Go Corporate vernance資本的變化 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告材料會計政策信息|304 |
| 估值計入綜合損益表或其他全面收益(虧損)表中的“其他營業收入”項下。 再鼎醫藥股份:根據國際財務報告準則第9號,本公司不可撤銷地選擇通過保監處將這項特定的 投資按公允價值指定為金融資產,因為參與並非出於 交易目的或收購方在業務組合中確認的或有對價 。投資在合併財務狀況表中記入“其他非流動資產”項下,公允價值變動在合併損益表和其他全面收益(虧損)表中記入“在FVTOCI指定的權益工具投資的公允價值 損益”項下。 2.14股東權益 權益工具是指在扣除實體所有負債後對其資產產生剩餘權益的任何合同。本公司發行的股權工具在扣除直接發行成本後按收到的收益確認。 本公司從未向其股東派發任何股息。截至2023年12月31日,沒有利潤可供分配。 截至2021年1月1日,公司將其本位幣和列報貨幣從歐元 改為美元。重新列報產生的差異被表示為折算 差異,這是股東權益中的一個組成部分。股本、股票溢價和其他準備金按交易日期的歷史匯率折算。 2.15基於股票的付款 向員工和其他提供類似服務的人以股權結算的基於股票的付款 按接受日期的股權工具的公允價值計量。股權結算 股份支付包括與本公司授予的股票期權和限制性股票單位有關的費用。 在接受股權結算的股份支付之日確定的公允價值是根據公司對最終將歸屬的股權工具的估計 在歸屬期間按直線計算的支出,並相應增加股本。在每個報告期結束時,本公司會修訂其對預期歸屬的權益工具數目的估計。如有修改原估計的影響,則在 合併損益表和其他 全面收益(虧損)的合併報表中確認,以使累計費用反映修訂後的估計,並對股權結算的股份支付準備金進行相應的調整。 股份支付費用記在“研發費用”或“銷售”項下。一般和行政費用“取決於每個受益人提供的服務的性質。 2.16所得税 合併損益表和其他綜合收益(虧損)的合併報表中的所得税代表當期税額和遞延税額的總和。 當期税額以當年的應税利潤為基礎。應税利潤不同於合併損益表和其他 綜合收益(虧損)表中報告的利潤,它不包括其他年度應納税或 應扣除的收入或費用項目,以及從未納税或扣除的項目。本公司的當期税項負債是根據報告period. ar Gr g oup enx Factors Risk Go Corporate vernance資本結束時已頒佈或實質性頒佈的税率計算的 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告材料會計政策信息|305 |
| 遞延税項乃根據綜合財務報表中資產及負債的賬面金額與計算應課税溢利時所採用的相應計税基準之間的暫時性差異而確認。遞延税項資產確認的範圍為: 很可能會有未來的應課税利潤,而這些可扣除的暫時性差額 可以用來抵銷。遞延税項資產的賬面金額於每個報告期結束時進行審核,並在不可能有足夠的 應課税利潤以收回全部或部分資產的情況下減少。遞延税項 如果存在法律上可強制執行的抵銷權利,則資產和負債被抵銷。 遞延税項資產和負債是根據報告期末已經頒佈或實質頒佈的税率(和税法),按預期在債務清償或資產變現期間適用的税率計量的。 本公司根據國際會計準則第12號使用兩步檢驗記錄不確定的税位 據此(1)本公司確定税位是否有可能被相關税務機關接受,以及(2)對於税務機關不可能全額接受的税務頭寸,公司根據具體的事實和情況,使用最有可能的金額或預期價值確認不確定的税收頭寸。 2.17產品淨銷售額 銷售商品的收入以反映公司預期有權在客户獲得對所交付貨物的控制權時有權獲得的對價的金額確認。這意味着客户有能力指導資產的使用。在與客户簽訂的合同中, 承諾的對價可以包括固定金額、可變金額或兩者兼有。對價金額可能會因折扣、返點、退貨、 退款或其他類似項目而有所不同。當確認的收入金額極有可能不會受到未來重大逆轉的影響時,或有對價計入交易價格 。 當我們根據IFRS 15收入確認準則在某一時間點履行履行義務時,即確認產品淨銷售額 與客户簽訂的合同收入 。 商業銷售產生的收入在“產品淨銷售額”項下的 綜合損益表和其他 全面收益(虧損)的綜合報表中列示。根據IFRS 15與客户簽訂的合同收入 ,根據與客户商定的交付和驗收條款,此類收入在產品實際轉讓時確認。 交易價款在客户獲得貨物的合法所有權時支付。 確認的收入金額反映了公司向客户提供的各種降價或退貨權利。此類降價和退貨權 符合IFRS 15與客户簽訂的合同收入的可變對價。 銷售的產品受各種政府和州計劃(如聯邦醫療保險和醫療補助)的保護,在這些計劃下,產品以折扣方式銷售。根據與某些客户的合同安排,向醫療保健機構提供返點。根據 特定合同安排,一些批發商 有權根據最終客户的銷售價格獲得退款獎勵。返點、按存儲容量使用計費和其他激勵措施在基礎銷售確認為產品sales. ar Gr g oup enx Factors Risk Go Corporate vernance減值的期間確認 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告材料會計政策信息|306 |
| The significant components of variable consideration are as follows: Co-payment assistance: We provide co-payment assistance to patients who have commercial insurance and meet certain eligibility requirements. We use the expected-value method for estimating co-payment assistance based on estimates of program redemption using data provided by third-party administrators. Estimates for the co-payment assistance are adjusted quarterly to reflect actual experience. We record an accrued liability for unredeemed co-payment assistance related to products for which control has been transferred to customers. Chargebacks: Chargebacks are discounts that occur when contracted parties purchase directly from a specialty distributor. Contracted parties, which currently consist primarily of Public Health Service Institutions and federal government entities purchasing via the Federal Supply Schedule, generally purchase the product at a discounted price. The specialty distributor, in turn, charges back the difference between the price initially paid by the specialty distributor and the discounted price paid to the specialty distributor by the contracted parties to the Company. The reserves for chargeback are based on known sales to contracted parties. We establish the reserves for chargebacks in the same period that the related revenue is recognized, resulting in an accrued liability and reduction of product gross sales. Rebates: We are subject to government mandated rebates for Medicaid Drug Rebate Program, Medicare Part D Prescription Drug Benefit Program, and other government health care programs in the U.S. Rebate amounts are based upon contractual agreements or legal requirements with public sector benefit providers. We use the expected-value method for estimating these rebates. The expected utilization of rebates is estimated based on third-party data from the specialty pharmacies and specialty distributor. Estimates for these rebates are adjusted quarterly to reflect the most recent information. We record an accrued liability and reduction of product sales for unpaid rebates related to products for which control has been transferred to customers. Medicare Part D Coverage Gap: The Medicare Part D coverage gap is a federal program to subsidize the costs of prescription drugs for Medicare beneficiaries in the U.S., which mandates manufacturers to fund a portion of the Medicare Part D insurance coverage gap for prescription drugs sold to eligible patients. Funding of the coverage gap is generally invoiced and paid in arrears. We estimate the impact of the Medicare Part D coverage gap using the expected-value method based on an amount expected to be incurred for the current quarter’s activity, plus an accrual balance for known prior quarters. Estimates for the impact of the Medicare Part D coverage gap are adjusted quarterly to reflect actual experience. We record an accrued liability for unpaid reserves related to the Medicare Part D coverage gap. Distributor fees: The specialty distributor provides distribution services to the Company for a fee, based on a contractually determined fixed percentage of sales. As the services being provided by the specialty distributor are not distinct, the recurring service fees paid to specialty distributors are treated as variable consideration and a reduction to the transaction price. We estimate these distributor fees and record such estimates in the same period the related revenue is recognized, resulting in a reduction of product gross sales. We record an accrued liability for unpaid distributor fees. Value-based arrangements (VBAs): VBAs are arrangements with third party payers where the Company will pay the third-party payers rebates and other fees on eligible purchases of the Company’s product. In consideration for the rebates and fees paid, the third-party Payers will cover its’ patient purchases made of the Company’s products. The structure of the rebates and fees are largely structured based on volume of product purchased. The rebates and fees paid to will be treated as variable consideration and a reduction to the transaction price. We use the expected-value method for estimating the ultimate rebate and fee paid, which are based on the volume of product sold. We apply the applicable rebate rate against a payer mix factor for the ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Material Accounting Policy Information | 307 |
| 相關患者羣體和在返點的生效計劃年度內銷售的瓶子,以 派生記錄的負債。這些協議的估計值每季度調整一次,以反映最新信息。我們記錄未付價值協議的應計負債。 上述估計金額在 合併損益表和 “產品淨銷售額”內的其他全面收益(虧損)合併表中確認為銷售總額的減少,並在 合併財務狀況表中的“貿易和其他應付款”中確認。它們將根據管理層可獲得的最新數據進行定期審查和適當調整。 以上每一項都需要大量估計、判斷和從外部來源獲得的信息。如果管理層的估計與實際結果不同,我們將記錄在調整期內可能影響產品銷售的 調整。 2.18合作和許可協議 公司目前有兩個正在進行的合作和許可協議,範圍為 IFRS 15: 再鼎醫藥 對於與再鼎醫藥的合作協議,公司評估存在不止一項明顯的履約義務,即轉讓許可並提供臨牀和商業產品 。公司得出結論認為,這些履約義務在合同範圍內是不同的。 因此,公司評估將交易價分配給所有確定的履約義務 。這兩個協議的交易價格由(I)固定的 部分組成,即以新發行的再鼎醫藥股票的形式預付,以及 有保證的、不可貸記的、不可退還的付款,以及(Ii)因在美國批准efgartigimod以及因提供臨牀和商業產品而收到的對價的里程碑付款。 交易價格的固定部分,以及efgartigimod在美國獲得批准的里程碑已分配給轉讓許可履行義務。公司 得出結論,截至合同生效日期(即2021年1月)的許可證具有 獨立價值。因此,本公司得出結論,向 再鼎醫藥授予許可的承諾是提供使用實體在授予許可時的知識產權的權利,因此,收入在2021年1月的某個時間點確認。 根據合作協議,本公司向ZAI 實驗室提供臨牀和商業供應。鑑於公司在交易中擔任 委託人,公司決定將此類銷售確認為收入,因為在將庫存轉移給再鼎醫藥之前,與庫存相關的風險由公司承擔。與臨牀供應有關的收入記錄在第 行“協作收入”項下。與商業供應相關的收入記錄在其他綜合收益(虧損)的合併報表中的第 項“產品淨銷售額”下。 與特許權使用費相關的收入記錄在第“協作收入”項下。 AbbVie 對於與AbbVie的協作協議,公司已確定轉讓許可證 與執行研發活動代表着 一項單一的履行義務。該公司的結論是,許可證在合同的上下文中並不明確。 交易價格由固定部分和可變部分組成,前者是預付許可費,後者是Research and ar Gr g oup enx Factors Risk Go Corporate vernance資本的里程碑付款和成本報銷 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告材料會計政策信息|308 |
| development activities delivered. Milestone payments are only included in the transaction price to the extent it is highly probable that a significant reversal in the amount of cumulative revenue recognition will not occur when the uncertainty associate with the variable consideration is subsequently resolved. Management estimates the amount to be included in the transaction price upon achievement of the milestone event. Sales-based milestones and sales-based royalties are a part of the Company’s arrangements but are not yet included in its revenues. The transaction price has been allocated to the single performance obligation and revenues has been recognized over the estimated service period based on an input model, being the percentage of completion method. The upfront license fee has been fully recognized since 2021 as the performance obligation has been fulfilled at that time. Milestone payments that become highly probable after the performance obligation has been fulfilled are therefore recognized at that point in time. 2.19 Cost of Sales Cost of sales are recognized when the associated revenue from product net sales is recognized. Cost of sales include material, manufacturing costs and other costs attributable to production, including shipping costs, as well as royalties payable on sold products. 3. Critical Accounting Estimates and Judgments In the application of the Company’s accounting policies, which are described above, the Company is required to make judgments, estimates and assumptions about the carrying amounts of assets and liabilities that are not readily apparent from other sources. The estimates and associated assumptions are based on historical experience and other factors that are considered to be relevant. Actual results may differ from these estimates. The estimates and underlying assumptions are reviewed on an ongoing basis. Revisions to accounting estimates are recognized in the period in which the estimate is revised if the revision affects only that period or in the period of the revision and future periods if the revision affects both current and future periods. Critical estimates in applying accounting policies Gross to net adjustments The product gross sales are subject to various deductions, which are primarily composed of rebates to government agencies, distributors, health insurance companies and managed healthcare organizations. These deductions represent estimates of the related obligations, requiring the use of judgment when estimating the effect of these sales deductions on product gross sales for a reporting period. These adjustments are deducted from product gross sales to arrive at product net sales. The significant components of variable consideration under revenue recognition policy summarizes the nature of these deductions and how the deduction is estimated, see note 2.17. After recording these, product net sales represent the Company’s best estimate of the cash that we expect to ultimately collect. If in future periods the actuals vary from prior period best estimates, this would affect revenue in the period of adjustment. Please refer to note 14 for the movement over the period and the ending balance of the gross-to-net-accruals. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Critical Accounting Estimates and Judgments | 309 |
| 4. Property, Plant and Equipment (in thousands of $) IT, office and lab equipment Right-of-use assets Buildings Right-of-use assets Vehicles Leasehold improve-ments Lease equipment Total Cost On January 1, 2021 4,889 11,721 2,273 1,424 346 20,653 Additions 3,163 4,923 802 543 – 9,430 Disposals (217) – – – – (217) Currency translation adjustment 104 (182) – 14 – (64) On December 31, 2021 7,938 16,462 3,075 1,981 346 29,802 Additions 962 3,353 905 – – 5,219 Disposals (105) – – – – (105) Currency translation adjustment (635) – – – – (635) On December 31, 2022 8,160 19,815 3,980 1,981 346 34,282 Additions 937 8,770 2,327 48 – 12,082 Disposals (202) – (757) (54) – (1,013) On December 31, 2023 8,895 28,585 5,550 1,975 346 45,350 Depreciation and impairment On January 1, 2021 (3,642) (4,044) (760) (543) (82) (9,071) Depreciation (1,118) (2,714) (651) (539) (34) (5,055) Disposals 158 – – – – 158 Currency translation adjustment 37 (15) – (11) – 10 On December 31, 2021 (4,565) (6,774) (1,411) (1,093) (116) (13,958) Depreciation (1,388) (2,179) (735) (257) (35) (4,593) Disposals 90 – – – – 90 Currency translation adjustment 408 5 1 1 – 414 On December 31, 2022 (5,454) (8,948) (2,145) (1,350) (150) (18,047) Depreciation (1,539) (2,839) (971) (189) (36) (5,574) Disposals 189 – 757 – – 946 On December 31, 2023 (6,804) (11,787) (2,359) (1,539) (186) (22,675) Carrying Amount On December 31, 2021 3,373 9,688 1,664 888 230 15,844 On December 31, 2022 2,706 10,867 1,835 631 196 16,234 On December 31, 2023 2,091 16,798 3,191 436 160 22,675 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Property, Plant and Equipment | 310 |
| Depreciation is recognized as from acquisition date onwards (unless asset is not ready for use) so as to write off the cost or valuation of assets less their residual values over their useful lives, using the straight-line method. Unless revised due to specific changes in the estimated useful life, annual depreciation rates are as follows: · Office and lab equipment: 3–5 years · IT equipment: 3 years As of December 31, 2023, there are no material commitments to acquire property, plant and equipment. Furthermore, no items of property, plant and equipment are pledged. See note 22 for information for leases where the Company is a lessee. 5. Intangible Assets (in thousands of $) Acquired R&D available for use Acquired In-Process R&D Software & databases Other Intangibles Total Cost On January 1, 2021 – 65,180 3,543 99,058 167,781 Additions – 5,000 – – 5,000 Translation differences – – (190) – (190) On December 31, 2021 – 70,180 3,353 99,058 172,591 Additions – – 992 102,000 102,992 Disposals – – (5) – (5) Derecognition – – – (99,058) (99,058) On December 31, 2022 – 70,180 4,340 102,000 176,519 Additions 56,000 – – – 56,000 Derecognition – – – (102,000) (102,000) Reclassification 52,931 (52,931) – – – On December 31, 2023 108,931 17,249 4,340 – 130,520 Amortization and impairment On January 1, 2021 – – (437) – (437) Amortization (470) – (470) On December 31, 2021 – – (907) – (907) Amortization – – (711) (99,058) (99,768) Derecognition – – – 99,058 99,058 On December 31, 2022 – – (1,618) – (1,618) Amortization (3,392) – (282) (102,000) (105,674) Derecognition – – – 102,000 102,000 On December 31, 2023 (3,392) – (1,900) – (5,292) ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intangible Assets | 311 |
| (in thousands of $) Acquired R&D available for use Acquired In-Process R&D Software & databases Other Intangibles Total Carrying Amount On December 31, 2021 – 70,180 2,446 99,058 171,684 On December 31, 2022 – 70,180 2,722 102,000 174,901 On December 31, 2023 105,539 17,249 2,440 – 125,228 Acquired In-Process R&D is mainly related to the in-licensing of the ENHANZE® drug delivery technology from Halozyme. In line with its accounting policies, the Company has capitalized the upfront payment upon commencement of the in-license agreement in 2019 and the development milestone payments when the respective milestone has been achieved. In June 2023, the Company obtained the FDA approval for VYVGART Hytrulo, which is a subcutaneous product combination of efgartigimod alfa and Halozyme’s ENHANZE® drug delivery technology. Upon this regulatory approval, the $52.9 million has moved from “Acquired In-Process R&D” to “Acquired R&D available for use”. Further, the additions to “Acquired R&D available for use” are related to regulatory and sales-based milestones triggered during 2023 related to the in-licensing of the ENHANZE® drug delivery technology from Halozyme. In line with its accounting policies, the Company has capitalized the regulatory and sales-based milestone payments when the respective milestones have been achieved. The “Acquired R&D available for use” are amortized under “Cost of sales” on a straight-line basis over their useful life, being the longer of the patent protection life of the Acquired R&D available for use and patent protection life of the combined product, which is 2036 for VYVGART Hytrulo. The Company performs an annual impairment review on the intangible assets. This review did not result in the recognition of an impairment charge for the years ended December 31, 2023, 2022 and 2021. In the fourth quarter of 2023, the Company utilized the PRV submitted with the sBLA filing for VYVGART Hytrulo for the treatment of CIDP, which resulted in amortization of $102.0 million of intangible asset which is recognized under “Research and development expenses” within the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss) and subsequent derecognition of $102.0 million of intangibles included under “other intangibles” on the consolidated statements of financial position. As of December 31, 2023, there are no material commitments to acquire intangible assets, except as set forth in note 29. No intangible assets are pledged as security for liabilities nor are there any intangible assets whose title is restricted. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Intangible Assets | 312 |
| 6.其他非流動資產 其他非流動資產包括通過損益或保監處按公允價值持有的非流動限制性現金和金融資產。 截至2023年12月31日,非流動限制性現金2,419 1,736 1,707非流動金融資產通過損益以公允價值持有 至保監處15,528 17,443 35,710其他非流動資產39,662 40,894 54,8762023年主要包括根據租賃協議為本公司的實驗室和辦公室支付的保證金 。 通過損益按公允價值持有的非流動金融資產包括AgomAb Treeutics NV的利潤 份額。2019年3月,本公司與AgomAb Treateutics NV簽訂了使用根據本公司免疫學創新計劃開發的模擬肝細胞生長因子簡單抗體™的許可證 。作為授予此許可證的交換,該公司獲得了AgomAb Treateutics NV的利潤份額。由於AgomAb Treateutics NV是一傢俬人公司,利潤份額的估值是基於級別3的假設。 2022年6月,AgomAb Treateutics NV獲得了3840萬歐元的B系列延期。該公司使用本輪B系列融資的資金後估值和 流通股數量來確定利潤分享工具的公允價值,這導致2022年10月通過損益記錄的非流動金融資產的公允價值發生變化 $430萬。 AgomAb Treateutics NV在C系列融資中獲得了1.0億美元。本公司的利潤份額隨着公司持有的股份數量的增加而稀釋,在AgomAb的貨幣後估值增加的情況下保持穩定,導致 非流動資產的公允價值不發生變化。 非流動金融資產的公允價值通過損益計入的公允價值變動在合併損益表和其他全面收益(虧損)的合併報表中確認。 作為大中國開發和商業化許可協議的一部分,公司於2021年獲得,其中,568,182股新發行的Zai Lab股票按每股132美元計算。權益工具於報告日期的公允價值乃參考該等證券於每個報告日期的收市價釐定(按公允價值等級分類為第1級)。本公司作出不可撤銷的選擇,在“FVTOCI”. ar Gr g oup enx Factors Risk Go Corporate vernance資本指定的股權工具投資的公平 價值收益/(虧損)”項下,不可撤銷地選擇通過保監處確認公允價值的後續變化 股票財務 審核報表 財務非財務 信息 其他非流動資產2023年年度報告|313 |
| 下表顯示了截至2023年12月31日、2022年12月31日、2022年12月31日和2021年12月31日的這些非流動金融資產的公允價值、損益或保險。 在12月31日, (千美元)2023 2022 2021年1月1日的成本76,659 76,659 1,659年1,659年1,659年1月1日的成本--12月31日的75,000美元公允價值調整(37,501)(23,490)4,648年1月1日的公允價值調整通過 損益進行的年內公允價值調整-4,256 11,152年公允價值調整通過OCI(1,915)(18,267)(39,290)(39,290) 12月31日的公允價值調整(39,416)(37,501)(23,490) 12月31日的公允價值調整淨值37,3158 53,169 7。 2023 2022 2021原材料和消耗品庫存240,836 126,046 70,134過程中的庫存47,074 65,016 37,705成品22,640 37,291 1,237總庫存310,550 228,353 109,076在綜合損益表和其他全面收益表中在銷售成本下確認的庫存成本在截至2023年12月31日的年度為1.012億美元(截至2022年12月31日的年度為2,940萬美元)。 等待工廠批准的投放前庫存為1.013億美元。 由於在其中一家等待批准的工廠於2022年生產的藥品 批次中檢測到2022年生產的藥品存在潛在缺陷,公司 減少了庫存,金額為4730萬美元。公司已獲得供應商的 承諾,將在未來幾年更換這些批次中的藥物,這一承諾反映在綜合財務狀況表中,金額為47.3Argenx資本 股票財務 審查報表 財務非財務 信息 million. ar Gr g oup enx Factors Risk Go Corporate vernance 2023年年報庫存|314 |
| 8.預付費用(當期) 本期預付費用由預付款組成,具體如下: 2023 2022 2021預付存貨22,460 11,667 10,786預付研發費用71,201 44,905 39,684預付廣告費用19,933 13,479 2,006預付軟件6,240 4,309 2,272其他預付費用14,238 1,662 4,198預付費用134,072 76,022 58,946 9.貿易及其他應收賬款由以下詳細信息組成:(單位:千美元)2023 2022 2021應收貿易賬面金額417,994 241,228 28,058應收利息13,126 12,918 1,325應收税金63,605 20,526 7,974其他應收賬款1,962 1,025 864貿易和其他應收賬款總額496,687 275,697 38,221貿易和其他應收賬款的賬面價值接近其各自的公允價值。 在2023,2022和2021年12月31日,我們沒有為預期信貸損失計提任何準備金。 有關財務風險management. ar Gr g oup enx Factors Risk Go Corporate vernance資本的更多信息,請參閲附註26 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告預付費用(當前)|315 |
| 10.金融資產-流動 這些流動金融資產涉及初始到期日超過3個月但不到12個月的定期賬户和不符合現金等價物的貨幣市場基金。 截至2023年12月31日,貨幣市場基金-46,162 73,052定期賬户1,131,000 1,345,646 929,000流動金融資產1,131,000 1,391,808 1,002,052流動金融資產包括2.21億美元(2億歐元) 於2023年12月31日,這可能在財務中產生外幣匯兑損益 結果根據美元/歐元匯率的波動,因為公司的 本位幣是美元。 有關財務風險管理的詳細信息,請參閲附註26。 11.現金和現金等價物 2023年12月31日 (以千美元為單位)貨幣市場基金1,678,100 669,147 997,092定期賬户350,54,116 95,090現金和現金等價物20,744 77,477 242,494總現金和現金等價物2,048,44 800,740 1,334,676現金和現金等價物可能包括現金和銀行餘額,儲蓄賬户,儲蓄賬户,現金等價物期限 原始到期日不超過3個月的賬户和貨幣市場基金 可隨時轉換為現金且價值變化風險微乎其微的貨幣市場基金。 現金頭寸與優先金融合作夥伴進行投資,這些合作伙伴大多被認為是信用評級良好的優質金融機構,以降低信用風險。 2023年12月31日,現金和現金等價物包括以歐元持有的7.028億美元(6.36億歐元) ,和以日元持有的820萬美元(人民幣11.646億元),可根據美元/歐元和美元/日元匯率的波動在財務業績中產生外幣匯兑損益 因為公司的本位幣是美元。 有關財務風險的更多信息,請參閲附註26 management. ar Gr g oup enx Factors Risk Go Corporate vernance資本{Br}共享財務 審核報表 財務非財務 信息 argenx 2023年年度報告財務資產-當前|316 |
| 12. Share Capital and Share Premium On December 31, 2023, the Company’s share capital was represented by 59,194,488 shares. All shares were issued, fully paid up and of the same class. The table below summarizes the share issuances as a result of offerings, exercise of stock options and the vesting of restricted stock units under the Company’s Employee Stock Option Plan. Roll forward of number of shares outstanding: Number of shares outstanding on January 1, 2021 47,571,283 Exercise of stock options 503,282 Global public offering in Euronext and Nasdaq on February 2, 2021 3,125,000 Over-allotment option exercised by underwriters on February 4, 2021 468,750 Number of shares outstanding on December 31, 2021 51,668,315 Exercise of stock options 1,024,626 Vesting of RSUs 19,581 Global public offering in Euronext and Nasdaq on March 23, 2022 2,333,334 Over-allotment option exercised by underwriters on March 29, 2022 350,000 Number of shares outstanding on December 31, 2022 55,395,856 Exercise of stock options 1,137,439 Vesting of RSUs 79,560 Global public offering in Nasdaq on July 18, 2023 2,244,899 Over-allotment option exercised by underwriters on July 19, 2023 336,734 Number of shares outstanding on December 31, 2023 59,194,488 On May 2, 2023, at the annual general meeting, the shareholders of the Company approved the authorization to the Board to issue up to a maximum of 10% of the then-outstanding share capital, for a period of 18 months. On July 18, 2023, argenx SE offered 2,244,899 of its ordinary shares through a global offering which consisted of 1,580,981 ADSs in the U.S. at a price of $490.0 per ADS, before underwriting discounts and commissions and offering expenses; and 663,918 ordinary shares in the European Economic Area at a price of €436.37 per share, before underwriting discounts and commissions and offering expenses. On July 19, 2023, the underwriters of the offering exercised their overallotment option to purchase 336,734 additional ADSs in full. As a result, argenx SE received $1.26 billion in gross proceeds from this offering, decreased by $65.9 million of underwriter discounts and commissions, and offering expenses, of which $0.8 million has been deducted from equity. The total net cash proceeds from the offering amounted to $1.2 billion. On December 31, 2023, an amount of €202,408.2, represented by 2,024,082 shares, still remained available under the authorization to issue shares as granted to the Board by the shareholders of the Company. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share Capital and Share Premium | 317 |
| 13. Share-Based Payments The Company has an equity incentive plan for the employees, key consultants, board members, senior management and key outside advisors (“key persons”) of the Company and its subsidiaries. In accordance with the terms of the plan, as approved by shareholders, employees may be granted stock options and/or restricted stock units. 13.1 Stock Option The stock options are granted to key persons of the Company and its subsidiaries. The stock options may be granted to purchase ordinary shares at an exercise price. The stock options have been granted free of charge. Each employee’s stock option converts into one ordinary share of the Company upon exercise. The stock options carry neither rights to dividends nor voting rights. Stock options may be exercised at any time from the date of vesting to the date of their expiry. The stock options granted vest, in principle, as follows: · 1/3rd of the total stock options granted will vest on the first anniversary of the granting of the stock options, and · 1/36th of the total grant on the first day of each month following the first anniversary of the date of grant of the stock options. Stock options granted to non-executive directors vest on the third anniversary of the date of grant. Upon leave of the key persons stock options must be exercised before the later of (i) 90 days after the last working day at argenx, or (ii) March 31 of the 4th year following the date of grant of those stock options, and in any case no later than the expiration date of the option. In order to prefinance the taxes that are paid upon the grant of stock options, Belgian employees have the ability, in exchange for the taxes due upon the grant of the stock options, to transfer the economic benefits related to part of those stock options to a third party. In the year ending December 31, 2023, the economic benefits of 43,336 stock options, for which accelerated vesting applies, were transferred to a third party. No other conditions are attached to the stock options. The following stock option arrangements were in existence during the current and prior years and which are exercisable at the end of each period presented: ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share-Based Payments | 318 |
| Exercise price per stock options (in $) 1) Outstanding stock options on December 31, Expiry date 2023 2022 2021 2022 2.70 – – 125,339 2024 2.70 3,308 19,743 94,088 2024 4.36 532 5,127 6,113 2024 7.92 81,500 214,800 276,500 2025 12.64 1,600 2,000 4,500 2025 10.46 99,326 101,861 105,857 2026 12.57 24,400 30,000 41,000 2026 12.67 97,972 99,772 102,840 2026 15.62 111,811 115,211 117,581 2027 20.34 38,434 42,509 53,143 2027 23.39 225,852 303,867 361,350 2023 89.31 – 12,111 85,080 2028 89.31 13,890 19,490 39,515 2023 95.38 – 124,338 321,473 2028 95.38 225,457 264,392 350,631 2024 125.40 26,171 110,774 111,174 2029 125.40 71,573 110,756 146,765 2024 150.00 104,176 202,852 203,658 2029 150.00 370,566 537,110 611,122 2025 132.08 16,712 16,712 16,712 2030 132.08 50,801 71,486 102,558 2025 216.75 126,331 127,731 129,711 2030 216.75 160,677 223,812 282,475 2025 221.24 31,424 32,100 32,100 2030 221.24 78,534 117,790 136,601 2030 273.60 559,173 620,014 692,214 2025 273.60 202,205 202,475 203,214 2026 259.01 23,491 23,491 24,366 2026 281.89 59,626 60,890 61,505 2026 286.75 45,228 45,862 48,138 2031 259.01 27,201 35,214 42,282 2031 281.89 128,600 167,406 207,464 2031 286.75 62,138 81,311 92,456 2026 341.67 80,425 80,833 82,430 2031 341.67 226,520 286,353 307,158 2027 312.16 13,957 14,976 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share-Based Payments | 319 |
| Exercise price per stock options (in $) 1) Outstanding stock options on December 31, Expiry date 2023 2022 2021 2032 312.16 58,255 79,155 – 2027 395.04 58,091 61,816 – 2032 395.04 192,291 238,532 – 2027 407.19 13,764 13,764 – 2032 407.19 73,288 85,199 – 2032 397.36 347,765 370,354 – 2027 397.36 136,459 137,778 – 2025 341.67 16,000 – – 2028 376.47 15,014 – – 2033 376.47 43,856 – – 2028 392.72 127,490 – – 2033 392.72 495,821 – – 2028 508.96 2,235 – – 2033 508.96 69,704 – – 2028–2032 2) 330.06 79,305 – – 5,118,949 5,511,767 5,619,113 1) Amounts have been converted to USD at the closing rate as of December 31, 2023. 2) In December 2023, the Company granted options for which the Belgian taxed beneficiaries had a 60-day period to choose between a contractual term of five or ten years. 2023 2022 2021 Number of stock options Weighted average exercise price 1) (in $) Number of stock options Weighted average exercise price 1) (in $) Number of stock options Weighted average exercise price 1) (in $) Outstanding at January 1 5,511,767 205.02 5,619,113 164.33 5,365,743 142.87 Granted 844,011 395.92 1,021,642 375.58 882,584 314.99 Exercised (1,137,439) 142.31 (1,025,780) 92.62 (503,282) 64.72 Forfeited (99,390) 356.57 (103,208) 273.93 (125,932) 234.98 Outstanding at December 31 5,118,949 255.41 5,511,767 205.02 5,619,113 164.33 Exercisable at December 31 3,030,486 179.22 3,983,960 148.11 3,613,371 106.53 1) Amounts have been converted to USD at the closing rate of the respective period. The weighted average share price at the date of exercise of options exercised during the year ended December 31, 2023 was $456.8, compared to $336.5 during the year ended December 31, 2022 and $305.9 during the year ended December 31, 2021. The weighted average remaining contractual life of the stock options outstanding amounted to 5.9 years on December 31, 2023 compared to 6.2 years on December 31, 2022 and 6.3 years on ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share-Based Payments | 320 |
| December 31, 2021. The table below shows the weighted average remaining contractual life for each range of exercise price: Exercise price (in $) Outstanding on December 31, 2023 Weighted average remaining contractual life (in years) 2.7–7.92 85,340 0.96 10.46–12.64 100,926 1.95 12.57–15.62 234,183 2.67 20.34–23.39 264,286 3.89 89.31–95.38 239,347 4.95 125.4–150 572,486 4.75 132.08–273.6 1,225,857 5.26 259.01–341.67 669,229 6.04 312.16–407.19 893,870 7.53 330.06–508.96 833,425 8.69 The fair market value of the stock options has been determined based on the Black and Scholes model using the following unobservable assumptions: · The expected volatility, determined on the basis of the implied volatility of the share price over the expected life of the option. · The expected option life, calculated as the estimated duration until exercise, taking into account the specific features of the plans. Below is an overview of the parameters used in relation to the determination of the fair value of the grants during 2023: Stock options granted in April 2023 July 2023 October 2023 December 20231) Number of options granted 61,056 629,121 74,529 79,305 Average Fair value of options (in $) 2) 158.21-196.18 176.44–271.59 123.94–209.04 161.88–165.69 Share price (in $) 2) 361.64-401.21 380.81–521.19 439.42–491.75 371.36 Exercise price (in $) 2) 370.34 387.35 485.01 329.26 Expected volatility 41.00–42.18% 36.22–43.99% 35.35–36.67% 36.20–36.21% Average Expected option life (in years) 4–6.50 4–6.50 4–6.50 6.15–6.50 Risk‑free interest rate 2.96–3.14% 2.90–3.03% 2.80–3.44% 2.40% Expected dividends –% –% –% –% 1) In December 2023, the Company granted a total of 79,305 stock options of which 8,459 stock options to Belgian taxed beneficiaries. Belgian taxed beneficiaries can choose between a contractual term of five or ten years. The expected option life ranges between 6.15 and 6.50 years. This estimate will be reassessed once the acceptance period of 60 days has passed and the beneficiaries will have made a choice between a contractual term of five or ten years. The total fair value of the grant to Belgian taxed beneficiaries would range from $ 1.1 million (100% of the stock options of Belgian taxed beneficiaries with a contractual term of five years) to $1.4 million (100% of the stock options of Belgian taxed beneficiaries with a contractual term of ten years). 2) Amounts have been converted to USD at the applicable rate prevailing at the grant date. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Share-Based Payments | 321 |
| 以下是與確定2022年期間授予的公允價值有關的參數概述: 2022年4月授予的股票期權2022年7月2022年10月授予的期權數量102,081 311,311 100,118 508,132期權的平均公允價值(美元)2)111.27-140.23 153.45-190.53 136.66-169.96 127.68-163.94股價(美元)2)320.84-321.06 378.11-397.92 352.97-376。01 368.69-377.61行權價(美元)2)312.22 372.69 359.80 381.97預期波動率39.18-40.87%41.30-43.10%39.64-45.97%39.74-40.26% 期權平均預期年限(年)4-6.504-6.504-6.50零風險利率1.05-1.62%1.77-2.28%2.57-2.80%3.09-3.29% 預期股息-% 1)2022年12月,公司共授予508,132份股票期權。比利時受益人可以選擇五年或十年的合同期限,以影響確定贈款公允價值時使用的參數。一旦超過受益人在五年或十年的合同期限、截至12月31日的財政年度使用的參數和公允價值之間做出選擇的60天承諾期, 2022年已重新評估。 2)金額已按授予日的適用匯率轉換為美元。 下面概述了與確定2021年期間授予的公允價值有關的參數: 2021年4月授予的股票期權2021年7月授予的股票期權2021年12月授予的期權數量67,833 280,339 144,824 389,588期權的平均公允價值(以美元為單位)1)98.96-154.88 131.65-159.13 101.53-131.80 75.03-145.34股價(美元)1)248.9-283.67 300.78-340.95 286.52-304.5 277.72-351.73行權價(美元)1)275.33 303.16 301.02 349.92預期波動率54.24-60.08%45.58-47.96%46.01-48.46%43.24-43.64% 期權平均預期壽命(年)4-6.50 4-6.50 4-6.50 4-650無風險利率(0.41)-(0.08%)%(0.41)-(0.17%)%(0.18)-(0.05)%0.03-0.67% 預期股息-% 1)金額已按相應期間的收盤價折算為美元。 截至 12月31日止年度,與股票期權有關的股份支付支出總額為1.64億美元。相比之下,截至2022年12月31日的年度為1.202億美元,截至2021年12月31日的年度為1.712億美元。 13.2限制性股票單位(RSU) 限制性股票單位授予公司及其子公司的關鍵人員。已免費授予RSU 。每位員工的RSU在歸屬後轉換為 公司的一股普通股。RSU既沒有分紅的權利,也沒有投票權。RSU 一旦轉換為普通股,可以從歸屬之日起隨時出售,沒有到期日,可以由參與者持有,但不限於。RSU的公允價值是基於公司普通股發行前一天的收盤價 。RSU在4年內授予,在grant. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告股票支付|322週年之際,授予總授予金額的1/4 |
| 本年度和前幾年存在以下限制性股票單位安排: 2023 2022 2021加權 平均 授予日期 公允價值(單位為 $) 平均 授予日期 加權 平均 授予日期 公允價值(單位為 $) 公允價值數量 加權 平均 公允價值(單位: $)Br}$) 1月1日的未歸屬單位385,280 387.20 213,038 314.25-- 授予192,237 396.22 243,010 375.81 216,522 313.84歸屬(105,678)352.61(53,872)- 沒收(29,517)358.49(16,896)307.11(3,484)288.92的未歸屬單位在 12月31 442,322 375.89 385,280 387.20 213,038 314.25的股份支付費用總額截至12月31日的一年,利潤或虧損總額為6900萬美元,相比之下,截至2022年12月31日的年度為3690萬美元,截至2021年12月31日的年度為810萬美元。 14.截至12月31日的貿易及其他應付款 , (千美元)2023 2022 2021貿易應付款245,557 188,721 208,850短期員工福利95,104 84,337 83,737毛淨應計項目55,788 19,478- 其他17,564 3,142 828貿易和其他應付款總額414,013 295,679 293,415貿易和其他應付款的賬面價值接近其各自的公允價值。 貿易應付款主要對應於臨牀和製造活動,包括與這些活動相關的應計費用。 短期員工福利包括應支付給公司員工的工資和獎金的應計款項和應計費用。 }ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 argenx 2023年年度報告貿易和其他應付款|323 |
| 下表彙總了截至2022年12月31日的年度毛淨應計項目的變動情況: (以千美元為單位) 回扣和 按存儲容量使用計費 經銷 費用、產品 返還和 截至1月1日的其他合計 餘額,2022- 與本年度銷售有關的當前估計35,426 10,740 46,166(與本年度銷售有關的貸項或付款)(20,028)(6,661)(26,689) 12月31日的餘額,2022 15,398 4,079 19,478與本年度銷售額相關的當前估計123,542 26,427 149,969上一年銷售額的調整(4,041)(883)(4,924) (與本年度銷售額有關的貸項或付款)(78,327)(20,722)(99,049) (與上一年度銷售額相關的貸項或付款)(6,910)(2,775)(9,685) 截至2023年12月31日的餘額49,662,126 55,788 (以千美元為單位)2023 2022 2021產品總銷售額1,342,148 446,923- 毛淨調整(151,365)(46,203)- 產品淨銷售額1,190,783 400,720- 截至2023年12月31日的12個月,產品淨銷售額與VYVGART和VYVGART SC的銷售額相關。在截至2022年12月31日的12個月內,產品淨銷售額與VYVGART的銷售額有關。 有關sale. ar Gr g oup enx Factors Risk Go Corporate vernance資本按國家/地區的產品淨銷售額細目,請參閲附註18 股票財務 審核報表 財務非財務 信息 Argenx 2023年年度報告產品淨銷售額|324 |
| 16.協作收入 下表按協作協議和收入類別彙總了截至 2023年12月31日、2022年和2021年12月31日的年度協作收入詳情: 預付款、里程碑付款、研發服務費和其他 收入。 截至12月31日的年度,[br}(千美元)2023 2022 2021再鼎醫藥--151,903強生--292,279 AbbVie--121預付款--444,303再鼎醫藥--25,634強生--22,865 AbbVie 30,000-102其他-5,365 1,214里程碑付款30,365 49,815強生--2,028其他-424 298研發服務費-424,326再鼎醫藥5,533 4,238 833其他協作收入5,533 4,238 833截至2023年12月31日,協作總收入35,533 10,026 497,277強生和艾伯維,如下所述。 再鼎醫藥 2021年1月6日,Argenx和再鼎醫藥宣佈了在中國大區開發和商業化efgartigimod的許可協議,授予再鼎醫藥 在大中國開發和商業化efgartigimod的獨家權利。 根據協議條款,公司將收到1.75億美元的合作付款,其中包括以568,182股新發行的再鼎醫藥股票的形式預付的7,500萬美元,按每股132美元計算,7,500萬美元作為保證不可貸記、不可償還的付款。2021年第一季度收到的,以及在美國食品和藥物管理局批准efgartigimod後額外支付的2,500萬美元里程碑付款。 本公司還有資格獲得基於efgartigimod在大中國地區的年淨銷售額而分階段收取的特許權使用費(按 百分比計算)。 正如與再鼎醫藥合作的會計政策中所述,本公司 得出結論,根據IFRS 15,本公司有兩項業績義務,即轉讓許可證和臨牀和商業產品的供應。這兩項協議的交易價格 由一個固定部分組成,即以新發行的再鼎醫藥股票的形式預付7,500萬美元,以及7,500萬美元的擔保,non-ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告合作收入|325 |
| Efgartigimod在美國獲得批准的價值2500萬美元的里程碑,以及臨牀和商業產品的供應所獲得的對價。交易價格的固定部分以及在美國批准efgartigimod的2,500萬美元里程碑已分配給轉讓許可證 履約義務,該義務已於合同生效日期 2021年1月滿足,因此收入在該時間點確認。 根據合作協議,該公司向再鼎醫藥提供臨牀供應。與臨牀供應相關的 收入記錄在 協作收入的“其他收入”項下。與版税相關的收入在 “協作收入”下記錄為“其他收入”。在截至2023年12月31日的年度內,確認了與商業供應和相關特許權使用費相關的第一筆收入。 有關 協議其餘要素的材料會計政策,請參閲附註2。 AbbVie 2016年4月,公司與AbbVie簽訂合作協議,開發 ARGX-115(ABBV-151)並將其商業化。 公司授予AbbVie獨家選擇權,在完成IND支持研究後的指定期限內,獲得全球範圍內的、獲得ARGX-115(ABBV-151)計劃的獨家許可,以開發和商業化產品。 2023年10月,該公司在啟動非關鍵臨牀試驗後實現了第二個開發里程碑,觸發了3,000萬美元的付款。 由於艾伯維ARGX-115(ABBV-151)的持續進展,本公司有資格 獲得未來開發、監管和商業里程碑付款,金額分別高達5,000萬美元、1.9億美元和3.25億美元。以及按個位數中位數到十幾歲以下的百分比分級 銷售版税, 按慣例扣減。 強生創新藥品 2021年6月4日,公司收到強生創新藥品(J&J)附屬公司Cilag GmbH 國際的終止通知,導致 聯合開發和商業化cuatuzumab的合作協議終止。終止後,公司得出結論認為,公司已基本履行了履行義務 ,因此,在截至2021年12月31日的12個月內記錄了3.151億美元。 17.截至12月31日的年度其他營業收入, (千美元)2023年2022年授予研發獎勵2,538 2,186 4,398研發獎勵27,815 19,502 13,970工資税退税11,925 8,576 12,621非流動金融資產公允價值變動-4,256 11,152其他營業收入42,278 34,520 42,141 ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務信息 argenx年度報告2023其他營業收入|326 |
| 17.1 Research and development incentives The Company has accounted for a tax incentive following a research and development tax incentive scheme in Belgium according to which the incentive will be refunded after a five year period, if not offset against the current tax payable over the period. 17.2 Payroll tax rebates The Company accounted for payroll tax rebates as a reduction in withholding income taxes for its highly qualified personnel employed in its research and development department. 18. Segment Reporting The Company manages its activities and operates as one business unit which is reflected in its organizational structure and internal reporting. The Company does not distinguish in its internal reporting different segments, neither business nor geographical segments. The chief operating decision‑maker is the Board of Directors. Following table summarizes the product net sales by country of sales based on the country of the entity that recognizes product net sales: Year Ended December 31, (in thousands of $) 2023 2022 United States 1,046,592 377,659 Japan 56,432 15,764 EMEA 72,852 7,297 China 14,907 – Total product net sales 1,190,783 400,720 The Company sells its products through a limited number of distributors and wholesellers. Four U.S. customers represent approximately 86% of the product net sales in U.S. during twelve months ended December 31, 2023 (compared to 91% for the same period in 2022). Collaboration revenue is generated by external customers with their main registered office geographically located as shown in the table below: Year Ended December 31, (in thousands of $) 2023 2022 2021 Denmark – 5,365 1,389 United States 30,000 – 317,396 China 5,533 4,238 178,370 Other – 424 123 Total collaboration revenue 35,533 10,026 497,277 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Segment Reporting | 327 |
| The property plant and equipment and intangible assets of the Company are geographically located as shown in the table below: At December 31, (in thousands of $) 2023 2022 1) 2021 1) Netherlands – – – Belgium 138,252 186,923 182,118 United States 6,219 2,275 3,091 Japan 2,971 1,938 2,319 Germany 461 130 – Total non-current assets 147,903 191,136 187,528 1) Prior year amounts were updated/recast to match current year presentation. 19. Research and Development Expenses Year Ended December 31, (in thousands of $) 2023 2022 2021 Personnel expenses 226,344 162,010 160,464 External research and development expenses 483,192 366,955 382,902 Materials and consumables 4,057 2,396 2,735 Depreciation and amortization 105,546 102,132 3,742 IT expenses 19,935 12,678 7,798 Other expenses 20,418 17,194 22,879 Total research and development expenses 859,492 663,366 580,520 20. Selling, General and Administrative Expenses Year Ended December 31, (in thousands of $) 2023 2022 2021 Personnel expenses 303,033 234,740 164,646 Marketing services 202,146 115,950 59,968 Professional fees 108,820 62,620 42,707 Supervisory board 8,362 6,912 12,958 Depreciation and amortization 2,366 2,211 2,126 IT expenses 20,408 17,431 8,977 Other expenses 66,770 32,268 16,263 Total Selling, general and administrative expenses 711,905 472,132 307,644 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Research and Development Expenses | 328 |
| 21. Personnel Expenses The personnel expenses mentioned in notes 19 and 20 above are as follows: Year Ended December 31, (in thousands of $) 2023 2022 2021 Short‑term employee benefits – Salaries 266,482 216,847 135,676 Short‑term employee benefits – Social Security 19,231 16,274 12,785 Post‑employment benefits 7,758 5,406 2,864 Termination benefits 1,089 401 818 Share‑based payment 226,830 151,912 167,965 Employer social security contributions stock options 7,987 5,910 5,002 Total personnel expenses 529,377 396,750 325,110 The post‑employment benefits relate to the pension plans the Company has in place for its employees. The average number of full‑time equivalents (FTE) employees by function is presented below: Year Ended December 31, Average Number of FTE 2023 2022 2021 Research and development 607.3 474.8 349.7 Selling, general and administrative 681.2 442.4 264.4 1,288.5 917.2 614.1 22. Leases The statements of financial position shows the following amounts relating to leases: Year Ended December 31, In thousands of $ 2023 2022 2021 Right-of-use assets Buildings 16,798 10,867 9,688 Vehicles 3,191 1,835 1,664 Equipment 160 196 230 20,149 12,897 11,583 Lease liabilities Current 4,646 3,417 3,509 Non-current 15,354 9,009 7,956 20,000 12,426 11,465 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Personnel Expenses | 329 |
| 截至2023年12月31日的年度,使用權資產的新增金額為1,110萬美元,而截至2022年12月31日和2021年12月31日的年度分別為420萬美元和570萬美元。 下表顯示了截至12月31日的租賃負債的到期日分析,2023年: (千美元) 少於 1年1-3年3-5年 超過 5年 合計 合同 現金流量 金額 租賃負債4,286,136 5,754 1,824,000綜合損益表和其他綜合收益(虧損)表顯示以下與租賃有關的金額: 截至12月31日的年度, 以千美元計的2023 2022 2021折舊費用 建築物2,839 2,179 2,714車輛971 735 651設備36 35 34 3,846 2,949 3,399利息支出(包括在財務成本中)693 1,343 412與短期租賃相關的支出1,517 732 212與上面未顯示為短期租賃的低價值資產租賃相關的支出 20 21 7 2023、2022和2021年租賃的現金流出總額為380萬美元,分別為420萬美元及 450萬美元。 本公司並無訂立任何以浮動租金或剩餘價值擔保的租賃協議。該公司的租約包括延期選擇權。這些選項 為管理租賃資產提供了靈活性,並與公司的業務需求保持一致。 公司判斷是否合理地確定擴展選項將是exercised. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 Argenx 2023年年報租賃|330 |
| 23.財務業績和匯兑收益/(虧損) 截至12月31日的年度 (千美元)2023 2022 2021利息收入92,962 24,741 3,489現金等價物和流動財務淨收益 通過損益按公允價值持有的資產 和現金等價物14,424 2,924 144財務收入107,386 27,665 3,633通過損益按公允價值持有的財務收入 和現金等價物(2)(1,713)(3,482) 其他財務費用(904)(2,193)(1,096) 財務費用(906)(3,906)(3,9064,578) 已實現匯兑收益/(虧損)29(3,743)15未實現匯兑收益/(虧損)14,044(28,989)(50,068) 匯兑收益/(虧損)14,073(32,732)(50,053) 截至12月31日的年度匯兑收益1,410萬美元,2023年主要是由於現金和現金等價物的未實現匯率收益,以及由於歐元/美元匯率在 期間的波動而以歐元計值的當前金融資產頭寸。所得税 在損益表中確認的所得税可以詳細説明如下: 截至12月31日的年度, (千美元)2023 2022 2021本年度(9,592)(27,162)(15,224) 所得税前幾年(2,080)(12)398當期税(費用)/福利(11,672)(27,174)(14,826) 臨時 差額21,115 46,894 6,304遞延税(費用)/福利21,115 46,894 6,304總税(費用)/福利9,443 19,720(8,522) ar Gr g oup enx Factors 風險 去 公司 Verance Capital 分享財務 審核報表 財務非財務 信息 argenx 2023年年度報告財務業績和匯兑損益|331 |
| 所得税準備與對未計提所得税準備的收入適用荷蘭法定税率所產生的金額之間的差額如下: 截至12月31日的年度, (千美元)2023 2022 2021税前虧損304,496 729,314 399,743所得税(費用)/福利按 適用納税年度的荷蘭法定聯邦所得税率計算1)78,560 188,163 99,936公司間資產交易/交易的影響396(112,200)- 在確定應納税結果時不可扣除的費用的影響 確定應納税結果(2,674)(1,570)(4,441) 在確定應納税時基於股份的支付費用的影響 結果(43,040)(27,043)(29,925) 在確定應納税結果時不應納税的股票發行費用的影響18,620 11,412 14,119優惠的影響87,123 18,263 13,413(去)確認遞延税項資產的影響 遞延税項資產變更對税損的影響(2,282)(194)(44,232) 公司經營的司法管轄區不同税率的影響(3,509)(5,566)(2,084) 變更(去)確認遞延税項資產的影響(124,457)(51,320)(50,389) 已支付的預扣税(68)-(5,076) (不足)/超額提供(2,080)(12)398其他2,854(213)(241) 確認的所得税(費用)/福利 綜合損益表9,443 19,720(8,522) 1)2022年和2023年的適用税率為25.8%,2022年期間,Argenx Benelux BV通過資產轉讓(下稱資產交易)將某些流水線活動轉移給Argenx BV,總金額為4.49億美元。 通過資產交易,Argenx Benelux BV實現了這一知識產權的資本收益 這導致利率調節項目分類為“公司間的影響 資產deal/transaction”. ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年年度報告所得税|332 |
| The available deferred tax assets relates to argenx US Inc., argenx UK Ltd and argenx Japan KK which are profitable due to the global transfer pricing model of argenx, and the deferred tax liabilities are related to argenx BV. The amount of deferred tax assets and liability by type of temporary difference can be detailed as follow: At December 31, 2023 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Accruals and allowances 13,189 – 13,189 Income tax benefit from excess tax deductions related to share-based payments 23,310 – 23,310 Profit in inventory 52,026 – 52,026 Other tax carryforwards 6,339 – 6,339 Property, plant and equipment 2,136 (1,550) 586 Non-current fixed assets – (5,155) (5,155) Other 1,760 – 1,760 Netting by taxable entity (1,549) 1,550 1 Net deferred tax assets/(liabilities) 97,211 (5,155) 92,056 At December 31, 2022 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Accruals and allowances 8,884 – 8,884 Income tax benefit from excess tax deductions related to share-based payments 26,887 – 26,887 Profit in inventory 29,711 – 29,711 R&D capitalized expense 11,316 – 11,316 Property, plant and equipment 856 (549) 307 Intangible assets – (3,430) (3,430) Non-current fixed assets – (4,975) (4,975) Other 2,117 – 2,117 Netting by taxable entity (549) 549 – Net deferred tax assets/(liabilities) 79,222 (8,406) 70,817 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Income taxes | 333 |
| At December 31, 2021 (in thousands of $) Assets Liabilities Net Deferred tax assets/(liabilities) Accruals and allowances 2,858 – 2,858 Income tax benefit from excess tax deductions related to share-based payments 26,026 – 26,026 Profit in inventory 3,305 – 3,305 Property, plant and equipment 532 (740) (208) Intangible assets – (2,714) (2,714) Non-current fixed assets – (3,725) (3,725) Other 210 – 210 Netting by taxable entity (740) 740 – Net deferred tax assets/(liabilities) 32,191 (6,438) 25,753 The change in net deferred taxes recorded in the consolidated statements of financial position can be detailed as follows: (in thousands of $) Deferred tax assets Deferred tax liabilities Balance at January 1, 2023 79,222 (8,406) Recognized in profit or loss 17,685 3,430 Recognized in equity 381 – Effects of change in foreign exchange rate (77) (179) Balance at December 31, 2023 97,211 (5,155) (in thousands of $) Deferred tax assets Deferred tax liabilities Balance at January 1, 2022 32,191 (6,438) Recognized in profit or loss 49,075 (2,180) Recognized in equity (1,960) – Effects of change in foreign exchange rate (84) 212 Balance at December 31, 2022 79,222 (8,406) (in thousands of $) Deferred tax assets Deferred tax liabilities Balance at January 1, 2021 15,038 (1,487) Recognized in profit or loss 11,385 (5,082) Recognized in equity 5,494 – Effects of change in foreign exchange rate 274 131 Balance at December 31, 2021 32,191 (6,438) ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Income taxes | 334 |
| 截至2023年12月31日,未使用税項損失的未確認遞延税金資產總額為1.961億美元,而2022年12月31日和2021年12月31日分別為1.893億美元和2.038億美元。該公司在2023年12月31日有7.833億美元的未使用税項虧損,而2022年12月31日和2021年12月31日分別為7.561億美元和7.896億美元。所有可結轉的税收損失都在比利時 (2022年12月31日為7.201億美元,2021年12月31日為7.207億美元)和荷蘭(2022年12月31日為3320萬美元,2021年12月31日為3540萬美元,2021年12月31日為2490萬美元), 根據適用的税法沒有到期日。 作為一家活躍在比利時的公司,我們預計將從創新收入扣除中受益,或IID,在比利時。創新收入扣除制度 允許對可歸因於專利產品等收入的淨利潤按低於其他收入的實際税率徵税。截至2023年、2022年和2021年底,我們在比利時的累計結轉IID分別為6.549億美元、4.288億美元和2.136億美元。截至2023年12月31日,IID的未確認遞延税資產為1.637億美元,而2022年12月31日為1.072億美元,2021年12月31日為5340萬美元。此外,比利時研發成本超額折舊的未確認遞延税資產於2023年12月31日為2.782億美元,而2022年12月31日為2.047億美元,2021年12月31日為1.663億美元(Argenx BV)。Argenx BV的未確認遞延税項資產在2023年12月31日達到1.063億美元,相比之下,2022年12月31日的知識產權資產未來攤銷為1.122億美元。 截至2023年12月31日,該公司估計有1.279億美元的未分配收益可歸因於外國子公司,這些收益沒有確認遞延税項負債撥備 因為公司可以控制扭轉 臨時差異的時間,並且在可預見的未來沒有分配計劃。 25.每股虧損 (以千美元為單位)2023 2022 2021本年度虧損(295,053)(709,594)(408,265) 加權平均發行股數 已發行股數57,169,253 54,381,371 51,075,827每股基本和攤薄(虧損)(以美元為單位)(5.16)(13.05)(7.99) 每股普通股收益/虧損除以該期間的虧損除以該年度內普通股的加權平均數量。 公司報告2023年淨虧損時,2022年和2021年,股票期權和RSU具有反稀釋效應,而不是稀釋效應。因此,基本的 和稀釋後的每股普通股虧損沒有區別 股份財務 審核報表 財務非財務 信息 share. ar Gr g oup enx Factors Risk Go Corporate vernance 2023年年度報告每股虧損|335 |
| 26.金融風險管理 金融風險集中管理。本公司協調國內和國際金融市場的准入,並持續考慮和管理與本公司活動有關的財務風險。這些風險涉及金融市場風險、信用風險、流動性風險和貨幣風險。由於公司沒有財務債務,因此不存在其他重要風險,如借款的利率風險。本公司不以投機為目的購買或交易金融工具。 金融資產和負債類別: 計量 12月31日賬面金額類別,[br}(千美元)2023 2022 2021金融資產-非流動FVTPL 21,715 21,715 17,459金融資產-非流動FVTOCI 15,528 17,443 35,710研發激勵 應收款-非流動攤銷成本76,706 47,488 32,707限制性現金-非流動攤銷成本2,419 1,736 1,707貿易和其他應收款攤銷成本496,687 275,697 38,221金融資產-流動FVTPL-46,162 73,052金融資產-流動攤銷成本1,131,1,345,646 929,147研究和開發激勵 應收賬款-當前攤銷成本2,584 1,578- 現金和銀行攤銷餘額2,419,777 242,707,707貿易和其他應收賬款攤銷成本496,687 275,697 38,221金融資產-流動FVTPL-46,162 73,052金融資產-流動攤銷成本1,131,1,345,646,929,147研究和開發獎勵 應收賬款-當前攤銷成本2,584 1,578- 現金和銀行餘額攤銷成本2,419 1,736 1,707,707貿易和其他應收賬款攤銷成本496,687 275,697 38,221金融資產-流動FVTPL-46,162 73,052金融資產-流動攤銷成本1,131,000 1,345,646,646 929,147研究和開發獎勵 應收賬款-當前攤銷成本2,584 1,578- 現金和銀行攤銷餘額2,419 1,736 1,707,707貿易和其他應收賬款攤銷成本496,687 275,697 38,221金融資產-流動FV現金等價物攤銷成本350,000 54,116 95,090貿易和其他應付款攤銷成本414,013 295,679 293,415貿易和其他應收賬款的賬面價值被視為與其公允價值相同,由於其短期性質。 通過損益或保險以公允價值持有的金融資產 通過損益或保險以公允價值持有的金融資產或通過保險以公允價值持有的金融資產由上市公司和非上市公司的股權工具以及貨幣市場基金組成。 本公司對出售該等股權工具沒有任何限制,且該等資產不在其任何負債項下質押。這些工具被歸類為通過損益或保監局按公允價值持有的金融資產,符合以下條件: ·關於流動金融資產和現金等價物的第一級公允價值計量,基於此類證券在每個報告日期的收盤價(資產淨值)。 ·關於非流動金融資產的第三級公允價值計量。 這些金融工具的市場價格可能面臨波動,並可能受到各種因素的影響,如全球經濟形勢。流動金融資產和現金等價物包括以歐元和美元提名的集合投資基金,其中基礎投資包括債券和其他國際債務證券。基於weighted ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年年度報告財務風險管理|336. |
| 標的工具的平均到期日,除其他外,這些投資被歸類為流動金融資產或現金等價物。 信用風險的最大敞口為報告日的賬面金額。 本公司分別於2023年12月31日、2023年12月31日、2022年12月31日和2021年12月31日按公允價值列賬以下資產: 於2023年12月31日,1,528-21,715級非流動金融資產15,528-21,715現金及現金等價物1,678,100- 於12月31日按公允價值列賬的資產1,693,628-21,715,2022年12月31日1級2級3非流動金融資產17,443-21,715流動金融資產46,162-- 現金及現金等價物669,147-- 截至2021年12月31日按公允價值入賬的資產732,752-21,715(以千美元為單位)1級2級3級非流動金融資產35,710-17,459流動金融資產73,052-- 現金及現金等價物997,092-- 披露日曆年度內按公允價值入賬的資產1,105,854-17,459在適用的類別之間沒有發生轉移。 非流動金融資產-3級。2019年3月,公司與AgomAb Treateutics NV 簽訂了使用根據公司的免疫學創新計劃開發的模擬肝細胞生長因子簡單抗體™的許可協議。作為授予許可證的交換條件,公司獲得了AgomAb Treateutics NV的利潤份額。 2021年3月,AgomAb Treateutics NV通過發行286,705股優先股獲得了7,400萬美元的B系列融資。本公司使用B系列融資的資金後估值和流通股數量來確定利潤分享工具的公允價值,導致計入損益的非流動金融 資產的公允價值變化為1,120萬美元。由於AgomAb Treateutics NV是一傢俬營公司,利潤份額的估值是基於級別3的假設。 2022年6月,由於B系列延期,profit-sharing ar Gr g oup enx Factors Risk Go Corporate vernance治療公司獲得了3,840萬歐元的資金支持。該公司使用本輪B系列融資的融資後估值和 確定AgomAb資本的公允價值時的流通股數量 股票財務 審查報表 財務非財務 信息 argenx 2023年年度報告財務風險管理|337. |
| instrument, which results in a change in fair value of non-current financial assets of $4.3 million recorded through profit or loss. In October 2023, AgomAb Therapeutics NV secured $100.0 million as a result of a Series C financing round. The Company’s profit share diluted as the number of shares held by the company stayed stable where the post-money valuation of AgomAb increased, which results in unchanged fair value of the non-current asset. Non-current financial assets – Level 1 In January 2021, as part of the license agreement for the development and commercialization for efgartigimod in Greater China (see note 16 for further information), the Company obtained, amongst others, 568,182 newly issued Zai Lab shares calculated at a price of $132 per share. The fair value of the equity instrument at period-end is determined by reference to the closing price of such securities at each reporting date (classified as level 1 in the fair value hierarchy), resulting in a change in fair value. The Company made the irrevocable election to recognize subsequent changes in fair value through OCI. Capital risk The Company manages its capital to ensure that it will be able to continue as a going concern. The capital structure of the Company consists of equity attributed to the holders of equity instruments of the Company, such as capital, reserves and accumulated losses as mentioned in the consolidated statements of changes in equity. The Company makes the necessary adjustments in the light of changes in the economic circumstances, risks associated to the different assets and the projected cash needs of the current and projected research activities. On December 31, 2023, cash and cash equivalents amounted to $2,048.8 million, current financial assets amounted to $1,131.0 million and total capital amounted to $5,658.6 million. The current cash situation and the anticipated cash generation and usage are the most important parameters in assessing the capital structure. The Company’s objective is to maintain the capital structure at a level to be able to finance its activities for at least 12 months. Cash income from existing and new partnerships is taken into account and, if needed and possible, the Company can issue new shares or enter into financing agreements. Credit risk Credit risk refers to the risk that a counterparty will default on its contractual obligations resulting in financial loss to the Company. The Company has adopted a policy of only dealing with creditworthy counterparties and obtaining sufficient collateral, where appropriate, as a means of mitigating the risk of financial loss from defaults. Concentrations in credit risk are determined based on an analysis of counterparties and their importance on the overall outstanding contractual obligations at year-end. The Company has a limited number of collaboration and license partners and therefore has a significant concentration of credit risk. However, it has policies in place to ensure that credit exposure is kept to a minimum and significant concentrations of credit exposure are only granted for short periods of time to high credit quality collaboration partners. The Company applied the IFRS 9 simplified approach to measuring expected credit losses which uses a lifetime expected loss allowance for all receivables. To measure the expected credit losses, receivables have been grouped based on credit risk characteristics and the days past due. The provision for expected credit losses was not significant given that there have been no credit losses over the last three years and the high quality nature of the Company’s customers. Cash and cash equivalents and current financial assets are invested with several highly reputable banks and financial institutions. The Company holds its cash and cash equivalents mainly with different banks which are independently rated with a minimum rating of ‘A-’. The ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Financial Risk Management | 338 |
| Company also holds cash equivalents in the form of money market funds with a recommended investment horizon of 6 months or shorter but with a low historical volatility. These money market funds are highly liquid investments, can be readily convertible into a known amount of cash. The company has adopted a policy whereby money market funds must have an average rating of “BBB” or higher. Liquidity risk The Company manages liquidity risk by maintaining adequate reserves, by continuously monitoring forecast and actual cash flows, and by matching the maturity profiles of financial assets and liabilities. The Company’s main sources of cash inflows are obtained through sale of commercial product, capital increases and collaboration agreements. This cash is invested in savings accounts, term accounts and short term investment funds in the form of money market funds. These money market funds represent the majority of the Company’s available sources of liquidity. Since all of these are immediately tradable and convertible in cash they have a no impact on the liquidity risk. Interest rate risk The only variable interest-bearing financial instruments are cash and cash equivalents and current financial assets. Changes in interest rates may cause variations in interest income and expense resulting from short-term interest-bearing assets. Interest rate cuts may have a negative impact on the interest income of the Company. For the year ended December 31, 2023, if applicable interest rates would increase/decrease by 25 basis points, this would have a positive/negative impact of $7.9 million (compared to $6.2 million for the year ended December 31, 2022 and $0.9 million for the year ended December 31, 2021). Foreign exchange risk The Company undertakes transactions denominated in foreign currencies, causing exposures to exchange rate fluctuations. The Company is mainly exposed to the Euro, Japanese yen, British pound and Swiss franc. To limit this risk, the Company attempts to align incoming and outgoing cash flows in currencies other than USD. The net exposure to exchange differences of the monetary assets (being cash, cash equivalents and current financial assets) of the Company at the end of the reporting period are as follows: At December 31, (in thousands of $) 2023 2022 2021 EUR 923,773 613,866 591,887 JPY 8,232 5,613 6,316 GBP 7 59,026 1,237 CHF 193 3,832 727 CAD 266 657 – SEK 1 7 – DKK 9 6 – ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Financial Risk Management | 339 |
| On December 31, 2023, if the euro would have strengthened/weakened versus the dollar by 10%, this would have had a negative/positive impact of $92.3 million, compared to $61.4 million and $53.8 million on December 31, 2022 and December 31, 2021, respectively. On December 31, 2023, if other currencies would have strengthen/weakened against the dollar by 10%, this would have had no significant impact. 27. Related Party Transactions 27.1 Relationship and transactions with joint venture entity In July 2022, the Company entered into a joint venture agreement with the University of Colorado Anschutz Medical Campus and UCHealth and created a separate legal entity, OncoVerity, Inc., which is focused on optimizing and advancing the development of cusatuzumab, a novel anti-CD70 antibody, in acute myeloid leukemia (AML). The Company contributed $2 million in 2022 and $13 million in 2023. The investment has been accounted under IAS 28 Investment in associates and joint ventures using the equity method of accounting and has been designated as “investment in joint venture” in the consolidated statements of financial position. The share of net loss resulting from investment in joint ventures is presented in consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss) in line “Loss from investment in joint ventures”. The cash contributions made by the Company to the Joint Venture is reported under Cash flow from investing activities under “Investment in joint venture”. 27.2 Relationship and transactions with subsidiaries See note 31 for an overview of the consolidated companies of the group, which are all wholly-owned subsidiaries of argenx SE. Balances and transactions between the Company and its subsidiaries, which are related parties of the Company, have been eliminated on consolidation and are not disclosed in this note. 27.3 Relationship and transactions with key personnel The Company’s key management personnel consists of the members of the management team and the members of the board of directors. Remuneration of key management personnel On December 31, 2023, the senior management consisted of 8 members: Chief Executive Officer, Chief Operating Officer, Chief Financial Officer, Chief Scientific Officer, General Counsel, Chief Medical Officer, Vice President Corporate Development and Strategy and Global Head of Quality Assurance. They provide their services on a full-time basis. On December 31, 2023, the board of directors consisted of 9 members: Peter Verhaeghe, Don deBethizy, Pamela M. Klein, A.A. Rosenberg, James M. Daly, Camilla Sylvest, Ana Cespedes, Steve Krognes and Tim Van Hauwermeiren. Only the Chief Executive Officer is a member of both the senior management team and the board of directors. The Chief Executive Officer does not receive any remuneration for his board membership, as this is part of his total remuneration package in his capacity as member of the senior management team. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Related Party Transactions | 340 |
| The remuneration package of the members of key management personnel comprises: Year Ended December 31, (in thousands of $, except for the number of stock options & RSUs) 2023 2022 2021 Remuneration of key management personnel Short-term benefits for senior management members as a group Gross salary 4,161 4,199 3,465 Variable pay 2,816 3,077 2,020 Employer social security 807 1,015 789 Other short term benefits 545 372 274 Termination Benefits – – 382 Post-employment benefits for senior management members as a group 167 104 150 Cost of stock options granted in the year for senior management members as a group 27,983 18,393 15,060 Cost of restricted stock units granted in the year for senior management members as a group 11,694 9,594 8,025 Employer social security cost related to stock options (494) 1,101 4,172 Total benefits for key management personnel 47,679 37,855 34,337 Numbers of stock options granted in the year Senior Management as a group 132,100 117,600 101,446 Numbers of restricted stock units granted in the year Senior Management as a group 30,425 26,500 22,888 Remuneration of non-executive directors Board fees and other short-term benefits for non-executive directors 533 437 435 Cost of stock options granted in the year for non-executive directors 2,280 3,643 3,263 Cost of restricted stock units granted in the year for non-executive directors 1,034 1,850 1,731 Total benefits for non-executive board members 3,847 5,929 5,429 Numbers of stock options granted in the year Non-executive directors 12,400 21,600 22,950 Numbers of restricted stock units granted in the year Non-executive directors 2,713 4,800 5,100 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Related Party Transactions | 341 |
| 其他 本公司或其任何附屬公司並無向董事會成員或高級管理層提供貸款、準貸款或其他擔保。我們並未與本公司的主要管理人員 達成交易,但如上文所述,有關他們作為高級管理層成員及董事會成員行使 授權的薪酬安排。 28.或有事項 公司目前沒有面臨任何可能對公司的綜合財務狀況產生重大不利影響的未決索賠或訴訟。 29。承諾 截至資產負債表日,尚未簽署收購物業、廠房和設備的承諾。 2019年2月,公司與Halozyme Treateutics,Inc.簽訂了全球合作和許可協議(經2020年9月修訂)。根據協議條款,公司將支付至多1.24億美元,以實現與VYVGART SC相關的特定監管 和基於銷售的里程碑。此金額表示如果實現所有里程碑將支付的最大 金額,但不包括基於單位銷售額的可變版税 付款。此外,公司將為每個目標支付1,250萬美元用於 未來目標提名,以及根據特定開發、法規和基於銷售的 里程碑的實現情況,為每個選定的目標支付最高1.6億美元的未來潛在付款,並根據額外的指定銷售里程碑的實現,為每個目標支付最高4,000萬美元。這一金額代表如果實現所有 個里程碑將支付的最高金額,但不包括基於單位銷售額的可變特許權使用費支付。 本公司與其藥物製造承包商Lonza的製造承諾涉及持續執行Efgartigimod的生物許可證申請(BLA)服務及其與未來潛在商業化相關的製造活動。 2018年12月,本公司與Lonza 簽署了第一份商業供應協議,涉及為Efgartigimod保留商業藥物物質供應能力。在 總額中,公司根據商業供應協議對efgartigimod的未償還承諾為3.618億美元。 在2022年間,公司與富士膠片簽署了一項協議,涉及大規模生產efgartigimod藥物物質的活動。總體而言,根據商業供應協議,公司對efgartigimod的未償還承諾為1,330萬美元。 截至2023年12月31日,該公司在banks. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告|342中獲得總計720萬美元的信貸額度 |
| 30. Audit Fees The following auditors’ fees were expensed in the consolidated statements of profit or loss and the consolidated statements of other comprehensive income (loss): Year Ended December 31, (in thousands of $) 2023 2022 2021 Audit fees 1) 1,979 1,394 1,183 Audit-related fees 330 380 267 Tax fees 2) – – 79 Total 2,309 1,774 1,529 1) Audit services performed by Deloitte Accountants B.V. as the external auditor referred to in Section 1 of the Dutch Accounting Firms Oversight Act (Wta) as well as by the Deloitte network. 2) Tax services performed by the Deloitte network. 31. Overview of Consolidation Scope The parent company argenx SE is domiciled in the Netherlands. The Company, argenx SE, has two subsidiaries, argenx BV and argenx Benelux BV, based in Belgium. argenx BV has ten subsidiaries. Details of the Company’s consolidated entities at the end of the reporting period are as follows: Name Country Participation argenx SE The Netherlands 100.00% argenx BV Belgium 100.00% argenx Benelux BV Belgium 100.00% argenx US, Inc. USA 100.00% argenx Switzerland, SA Switzerland 100.00% argenx Japan KK Japan 100.00% argenx France SAS France 100.00% argenx Germany GmbH Germany 100.00% argenx Canada Inc. Canada 100.00% argenx UK Ltd. United Kingdom 100.00% argenx Netherlands Services B.V. The Netherlands 100.00% argenx Italy S.r.l. Italy 100.00% argenx Spain S.L. Spain 100.00% 32. Events After the Balance Sheet Date No events have occurred after the balance sheet date that could have a material impact on the consolidated financial statements. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Audit Fees | 343 |
| Company Financial Statements for argenx SE for the Year ended December 31, 2023 Signatures of Executive and Non-Executive Directors In accordance with article 2:101 of the Dutch Civil Code, the annual accounts were signed by all executive and non-executive directors on March 19, 2024. 6.3 6.3.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Financial Statements | 344 |
| Company Financial Statements for argenx SE For argenx SE For the year ended December 31, 2023 Company Balance Sheet on December 31, 2023 argenx SE At December 31, (In thousands of $) Note 2023 2022 Assets Non-current assets Financial fixed assets 2 Investments in Group Companies 3,703,280 2,583,759 Other financial assets 1 1 Total financial fixed assets 3,703,281 2,583,760 Total non-current assets 3,703,281 2,583,760 Current assets Receivables 3 369,640 140,185 Cash and cash equivalents 4 28,744 92,096 Total current assets 398,384 232,281 Total assets 4,101,665 2,816,041 Equity and liabilities Equity 5 Share capital 7,058 6,640 Share premium 5,651,497 4,309,880 Accumulated losses (2,404,845) (2,109,791) Reserve for share-based payments 749,324 515,158 Translation reserves 131,543 129,280 Other reserves (37,073) (37,467) Total equity 4,097,505 2,813,699 Current liabilities 6 Accounts payable 266 20 Intercompany payables 2,127 1,130 Taxes payable 925 155 Accrued expenses 841 474 Other payables 0 563 Total liabilities 4,159 2,342 Total equity and liabilities 4,101,665 2,816,041 6.3.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Company Financial Statements for argenx SE | 345 |
| Company Profit and Loss Account for the Year Ended December 31, 2023 argenx SE Year Ended December 31, (In thousands of $) Note 2023 2022 Intercompany recharges 0 0 Total operating income 0 0 G&A expenses (19,303) (15,543) Total operating expenses (19,303) (15,543) Operating result (19,303) (15,543) Financial income and expense 7 19,378 344,696 Share in result of subsidiaries 8 (294,476) (1,038,746) Result before taxation (294,402) (709,594) Taxation on result of ordinary activities (652) 0 Result after taxation (295,053) (709,594) Notes to the Company Financial Statements of argenx SE 1. Accounting Information and Policies 1.1 Basis of Preparation The company financial statements of argenx SE (hereafter: the company) have been prepared in accordance with Part 9, Book 2 of the Dutch Civil Code. In accordance with article 362 sub8, Book 2 of the Dutch Civil Code, the company’s financial statements are prepared based on the accounting principles of recognition, measurement and determination of profit, as applied in the consolidated IFRS financial statements. 1.2 Summary of Significant Accounting Policies In case no other policies are mentioned, refer to the accounting policies as described in the summary of material accounting policies in the consolidated IFRS financial statements. For an appropriate interpretation, the company financial statements of argenx SE should be read in conjunction with the consolidated IFRS financial statements. Participating Interests in Group Companies Participating interests in group companies are valued using the equity method, applying the IFRS accounting policies endorsed by the European Union. Following the adoption of IFRS 9 by the group, and our interpretation of the Dutch Accounting Standard 100.108, the company shall, upon identification of a credit loss on an intercompany loan and/or receivable, eliminate the carrying amount of the intercompany loan and/or receivable for the value of the identified credit loss. Result of Participating Interests The share in the result of participating interests consists of the share of the Company in the result of these participating interests. In so far as gains or losses on transactions involving the transfer of assets and liabilities between the Company and its participating interests or between participating interests themselves can be considered unrealized, they have not been recognized. 6.3.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 346 |
| All amounts are presented in thousands of USD, unless stated otherwise. The balance sheet and income statement references have been included. These refer to the notes. 1.3 Change in Functional and Presentation Currency as of January 1, 2021 As of January 1, 2021, the Company changed its functional and presentation currency from EUR to USD. The change in functional currency was made to reflect that USD has become the predominant currency in the Company, representing a significant part of the Company’s cash flows and financing. The change has been implemented with prospective effect. 2. Financial Fixed Assets The Company has two Belgian subsidiaries, argenx BV, which carries out the research and development activities of the Group and argenx Benelux BV, which, as of 2023, is a commercial company that will handle the commercial activities within the Benelux area. Argenx Benelux BV was incorporated through a partial demerger of argenx BV in 2020. On December 27, 2022, argenx Benelux BV transferred certain pipeline activities to argenx BV through a transfer of assets, (hereafter referred to as “asset deal”), for a total amount of $449 million. As a result of the asset deal, argenx Benelux BV realized a capital gain. argenx Benelux BV has distributed an interim dividend of EUR 325 million to argenx SE, which in turn has increased the share capital of argenx BV for $345 million. Argenx BV has ten subsidiaries, argenx US Inc., argenx Japan KK, argenx Switzerland SA, argenx Germany GmbH, argenx France SAS, argenx Canada Inc., argenx Netherlands Services BV, argenx UK Ltd, argenx Italy SRL, argenx Spain SL. The financial fixed assets mainly consist of the 100% participations in argenx BV and argenx Benelux BV, both registered at Industriepark 7, Zwijnaarde, Belgium. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 347 |
| The movement in financial fixed assets is as follows: At December 31, (in thousands of $) 2023 2022 Investments in Group Companies Opening balance 2,583,759 2,386,238 Share of loss of Investments (294,476) (1,038,746) Share-based payment expenses of investments 228,023 153,169 Fair Value gain on Financial Assets at FVTPL (67,200) (44,229) Capital increase subsidiaries 1,262,653 1,149,907 Changes booked directly in equity at subsidiary level (9,480) (22,580) Closing balance 3,703,279 2,583,759 Receivable/(payable) on Group companies 0 0 Investments in Group companies 3,703,279 2,583,759 Other financial assets Opening balance 1 1 Balance as at year-end 1 1 Total financial fixed assets 3,703,280 2,583,760 3. Receivables At December 31, (in thousands of $) 2023 2022 Interest receivable 133 323 Other receivables 368,543 138,918 Prepaid expenses 964 943 Total receivables 369,640 140,185 Receivables fall due in less than one year. The fair value of the receivables approximates the nominal value, due to their short-term character. 4. Cash and Cash Equivalents At December 31, (in thousands of $) 2023 2022 Money market funds 28,736 91,002 Current bank accounts 8 1,094 Total cash in banks 28,744 92,096 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 348 |
| 5. Equity (in thousands of $) Share capital Share premium Retained earnings Share based payment reserves Other reserves Translation reserves Total equity Equity per 31 December 2021 6,233 3,462,775 (1,400,197) 356,875 (23,146) 131,684 2,534,224 Result of the year 0 0 (709,594) 0 0 (709,594) SBP result 0 0 0 158,282 0 158,282 Capital increase 294 759,878 0 0 0 760,172 Exercised stock options 113 93,082 0 0 0 93,195 Changes booked directly in equity at subsidiary level 0 (5,855) 0 0 (14,321) (2,404) (22,580) Equity per 31 December 2022 6,640 4,309,880 (2,109,791) 515,158 (37,467) 129,280 2,813,699 Result of the year 0 0 (295,053) 0 0 (295,053) SBP result 0 0 0 234,167 0 234,167 Capital increase 288 1,195,623 0 0 0 1,195,910 Exercised stock options 130 158,133 0 0 0 158,263 Changes booked directly in equity at subsidiary level 0 (12,138) 0 0 395 2,263 (9,480) Equity per 31 December 2023 7,058 5,651,497 (2,404,845) 749,324 (37,073) 131,543 4,097,505 For the details on Share based payments we refer to note 13 of the consolidated IFRS financial statements. The company holds no legal reserves as part of the equity. 6. Current Liabilities At December 31, (in thousands of $) 2023 2022 Accounts payable 266 20 Intercompany payables 2,127 1,130 Taxes payable 925 155 Accrued expenses 841 474 Other payables 0 563 Total current liabilities 4,159 2,342 All current liabilities fall due in less than one year. The fair value of the current liabilities approximates the nominal value, due to their short-term character. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 349 |
| 7. Financial Result and Exchange Gains/(Losses) At December 31, (in thousands of $) 2023 2022 Interest income on bank deposits 0 2 Net gains on investments at FVTPL 7,343 1,151 Fees collected from ADS holders 500 466 Interest on I/C current account 3,893 321 Dividend income 0 345,784 Financial income 11,736 347,724 Net losses on investments at FVTPL 0 0 Interest expense 0 (199) Other financial expenses (29) (143) Financial expenses (29) (342) Exchange gains/(losses) 7,671 (2,686) Financial income and expense 19,378 344,696 8. Share in Result of Subsidiaries The Company has two Belgian subsidiaries, argenx BV, which carries out the research and development activities of the Group and argenx Benelux BV, which, as of 2023, is a commercial company that will handle the commercial activities within the Benelux area. Year ended December 31, (in thousands of $) 2023 2022 argenx BV (307,191) (562,594) argenx Benelux BV 12,656 (476,152) (294,476) (1,038,746) 9. Other Disclosures Contingent Liabilities The contingent liabilities of the Company consist of a rental agreement for office space in Amsterdam for an amount of KUSD 9 per annum. The lease contract has a duration of two years. Related-Party Transactions All legal entities that can be controlled, jointly controlled or significantly influenced are considered as a related party. Also, entities which can control the company are considered a related party. In addition, directors, other key management of argenx SE and close relatives are regarded as related parties. Other than the intercompany cross-charges, there were no related party transactions. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 350 |
| Remuneration Remuneration of executive director for 2023 and 2022 is as follows: (in $) 2023 2022 Base salary 655,787 638,901 Short term incentive 590,215 766,682 Option awards 8,084,605 4,174,684 Restricted stock units 2,575,174 2,159,689 Pension contributions 22,821 23,384 Other 16,232 14,958 Total remuneration executive director 11,944,835 7,778,298 Part of the remuneration of the executive director is being paid by subsidiaries of argenx SE. See note 27 of the notes to the consolidated IFRS financial statements for the remuneration of non-executive Board of directors. Information Relating to Employees During the year 2023, the Company had an average of 0.2 FTE (2022: 0.2 FTE). Auditor’s Fees See note 30 of the notes to the consolidated IFRS financial statements. Proposal for Appropriation of the Result The Company reported a net loss of $295.1 million for the year ended on December 31, 2023. The Board of Directors proposes to carry forward the net loss of the year 2023 to the accumulated losses. Anticipating the approval of the financial statements by the shareholders at the annual general meeting of shareholders, this proposal has already been reflected in the 2023 financial statements. Events after the balance sheet date For the events after balance sheet date, we refer to note 32 of the consolidated IFRS financial statements. Amsterdam, March 21, 2024 The Director Tim Van Hauwermeiren, CEO ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Notes to the Company Financial Statements | 351 |
| Other Information Provision in the articles of association governing the appropriation of results 1. The company shall have a policy on reserves and dividends which shall be determined and may be amended by the board of directors. The adoption and thereafter each material change of the policy on reserves and dividends shall be discussed at the general meeting under a separate agenda item. 2. From the profits, shown in the annual accounts, as adopted, the board of directors shall determine which part shall be reserved. Any profits remaining thereafter shall be at the disposal of the general meeting. The board of directors shall make a proposal for that purpose. A proposal to pay a dividend shall be dealt with as a separate agenda item at the general meeting. 3. Distribution of dividends on the shares shall be made in proportion to the nominal value of each share. 4. Distributions may be made only insofar as the company’s equity exceeds the amount of the paid in and called up part of the issued capital, increased by the reserves which must be kept by virtue of the law. 5. If a loss was suffered during any one year, the board of directors may resolve to offset such loss by writing it off against a reserve which the company is not required to keep by virtue of the law. 6. The distribution of profits shall be made after the adoption of the annual accounts, from which it appears that the same is permitted. 7. The board of directors may, subject to due observance of the policy of the company on reserves and dividends, resolve to make an interim distribution, provided the requirement of paragraph 4 of this article has been complied with, as shown by interim accounts. Such interim accounts shall show the financial position of the company not earlier than on the first day of the third month before the month in which the resolution to make the interim distribution is announced. Such interim accounts shall be signed by all members of the board of directors. If the signature of one or more of them is missing, this shall be stated and reasons for this omission shall be given. The interim accounts shall be deposited in the offices of the trade register within eight days after the day on which the resolution to make the interim distribution has been announced. 8. At the proposal of the board of directors, the general meeting may resolve to make a distribution on shares wholly or partly not in cash but in shares. 9. The board of directors may, subject to due observance of the policy of the company on reserves and dividends, resolve that distributions to holders of shares shall be made out of one or more reserves. 10. A claim of a shareholder for payment of a distribution shall be barred after five years have elapsed. 6.3.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Other Information | 352 |
| Independent Auditor’s Report To the shareholders and the Board of Directors of argenx SE Report on the audit of the financial statements for the year ended December 31, 2023 included in The Annual Report Our opinion We have audited the financial statements for the year ended December 31, 2023 of argenx SE, based in Rotterdam, the Netherlands. The financial statements comprise the consolidated financial statements and the company financial statements. In our opinion: · The accompanying consolidated financial statements give a true and fair view of the financial position of argenx SE as at December 31, 2023, and of its result and its cash flows for 2023 in accordance with International Financial Reporting Standards as adopted by the European Union (EU-IFRS) and with Part 9 of Book 2 of the Dutch Civil Code. · The accompanying company financial statements give a true and fair view of the financial position of argenx SE as at December 31, 2023, and of its result for 2023 in accordance with Part 9 of Book 2 of the Dutch Civil Code. The consolidated financial statements comprise: 1. The consolidated statements of financial position as at December 31, 2023. 2. The following statements for 2023: the consolidated statements of profit or loss, the consolidated statements of comprehensive income (loss), the consolidated statements of cash flows and the consolidated statements of changes in equity. 3. The notes comprising material accounting policy information and other explanatory information. The company financial statements comprise: 1. The company balance sheet as at December 31, 2023. 2. The company profit and loss account for 2023. 3. The notes comprising a summary of the accounting policies and other explanatory information. Basis for our opinion We conducted our audit in accordance with Dutch law, including the Dutch Standards on Auditing. Our responsibilities under those standards are further described in the 'Our responsibilities for the audit of the financial statements' section of our report. We are independent of argenx SE in accordance with the EU Regulation on specific requirements regarding statutory audit of public-interest entities, the Wet toezicht accountantsorganisaties (Wta, Audit firms supervision act), the Verordening inzake de onafhankelijkheid van accountants bij assurance-opdrachten (ViO, Code of Ethics for Professional Accountants, a regulation with respect to independence) and other relevant independence regulations in the Netherlands. Furthermore, we have complied with the Verordening gedrags- en beroepsregels accountants (VGBA, Dutch Code of Ethics). We believe the audit evidence we have obtained is sufficient and appropriate to provide a basis for our opinion. 6.3.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 353 |
| Information in support of our opinion We designed our audit procedures in the context of our audit of the financial statements as a whole and in forming our opinion thereon. The following information in support of our opinion was addressed in this context, and we do not provide a separate opinion or conclusion on these matters. Materiality Based on our professional judgement we determined the materiality for the financial statements as a whole at USD 50,000,000. The materiality is based on 3.5% of operating expenses excluding cost of sales and excluding the loss from investment in joint venture. We have also taken into account misstatements and/or possible misstatements that in our opinion are material for the users of the financial statements for qualitative reasons. We agreed with the Board of Directors that misstatements in excess of USD 2,500,000, which are identified during the audit, would be reported to them, as well as smaller misstatements that in our view must be reported on qualitative grounds. Scope of the group audit argenx SE is at the head of a group of entities. The financial information of this group is included in the consolidated financial statements of argenx SE. Because we are ultimately responsible for the opinion, we are also responsible for directing, supervising and performing the group audit. In this respect we have determined the nature and extent of the audit procedures to be carried out for group entities. The audit procedures on all group entities have been performed by the group engagement team without using the work of other auditors. By performing the procedures mentioned above at group entities, together with additional procedures at group level, we have been able to obtain sufficient and appropriate audit evidence about the group's financial information to provide an opinion on the consolidated financial statements. Audit approach fraud risks We identified and assessed the risks of material misstatements of the financial statements due to fraud. During our audit we obtained an understanding of the entity and its environment and the components of the system of internal control, including the risk assessment process and management's process for responding to the risks of fraud and monitoring the system of internal control and how the Board of Directors exercises oversight, as well as the outcomes. We evaluated management’s fraud risk assessment. We evaluated the design and relevant aspects of the system of internal control and in particular the fraud risk assessment, as well as among others the code of conduct, whistle blower procedures and incident registration. We evaluated the design and the implementation and, where considered appropriate, tested the operating effectiveness, of internal controls designed to mitigate fraud risks. As part of our process of identifying fraud risks, we evaluated fraud risk factors with respect to financial reporting fraud, misappropriation of assets and bribery and corruption in close co-operation with our forensic specialists. We evaluated whether these factors indicate that a risk of material misstatement due to fraud is present. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 354 |
| We identified the following fraud risk and performed the following specific procedures: We identified a risk of material misstatement due to fraud related to revenue recognition. The risk exists that the Company did not accurately record the Gross-to-Net adjustments because of a materially incorrect estimation of the payer mix, aggregate value based agreement (VBA) rates or payer mix constraint. Reference is made to the section 'Our key audit matters' for our procedures performed. We furthermore identified a risk of material misstatement due to fraud related to management override of controls. Management is in a unique position to perpetrate fraud because of management’s ability to manipulate accounting records and prepare fraudulent financial statements by overriding controls that otherwise appear to be operating effectively. We tested the appropriateness of journal entries recorded in the general ledger and other adjustments made in the preparation of the financial statements. In addition, we assessed whether there were any significant unusual transactions outside the normal course of business. We incorporated elements of unpredictability in our audit. We also considered the outcome of our other audit procedures and evaluated whether any findings were indicative of fraud or non-compliance. We considered available information and made enquiries of relevant management team members, (including the Chief Executive Officer, Chief Operating Officer, Chief Financial Officer, General Counsel, Global Head of Quality, and Chief Medical Officer) and the Board of Directors. We evaluated whether the selection and application of accounting policies by the group, particularly those related to subjective measurements and complex transactions, may be indicative of fraudulent financial reporting. We evaluated whether the judgments and decisions made by management in making the accounting estimates included in the financial statements indicate a possible bias that may represent a risk of material misstatement due to fraud. Management insights, estimates and assumptions that might have a major impact on the financial statements are disclosed in note 3 of the financial statements (Critical accounting estimates and judgments). We performed a retrospective review of management judgments and assumptions related to significant accounting estimates reflected in prior year financial statements. We evaluated the reasonableness of management’s estimates with respect to the gross-to-net adjustments for product net sales in the United States of America. Reference is made to the section 'Our key audit matters'. For transactions of interest, for instance in relation to donations to patient charities, we evaluated whether the business rationale of the transactions suggests that they may have been entered into to engage in activities in relation to bribery and corruption. This did not lead to indications for fraud potentially resulting in material misstatements. Audit approach compliance with laws and regulations We assessed the laws and regulations relevant to the Company through discussion with the General Counsel and the Head of Global Quality, reading minutes and reports of internal audit. We involved our forensic specialists in this evaluation. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 355 |
| As a result of our risk assessment procedures, and while realizing that the effects from non-compliance could considerably vary, we considered the following laws and regulations: (corporate) tax law, the requirements under the International Financial Reporting Standards as adopted by the European Union (EU-IFRS) and Part 9 of Book 2 of the Dutch Civil Code with a direct effect on the financial statements as an integrated part of our audit procedures, to the extent material for the financial statements. We obtained sufficient appropriate audit evidence regarding provisions of those laws and regulations generally recognized to have a direct effect on the financial statements. Apart from these, argenx SE is subject to other laws and regulations where the consequences of non-compliance could have a material effect on amounts and/or disclosures in the financial statements, for instance, through imposing fines or litigation. Given the nature of argenx SE's business and the complexity of healthcare regulations, there is a risk of non-compliance with the requirements of such laws and regulations. In addition, we considered major laws and regulations applicable to listed companies. Our procedures are more limited with respect to these laws and regulations that do not have a direct effect on the determination of the amounts and disclosures in the financial statements. Compliance with these laws and regulations may be fundamental to the operating aspects of the business, to argenx SE’s ability to continue its business, or to avoid material penalties (e.g., compliance with healthcare regulations) and therefore non-compliance with such laws and regulations may have a material effect on the financial statements. Our responsibility is limited to undertaking specified audit procedures to help identify non-compliance with those laws and regulations that may have a material effect on the financial statements. Our procedures are limited to (i) inquiry of management, the Board of Directors, and others within argenx SE as to whether argenx SE is in compliance with such laws and regulations and (ii) inspecting correspondence, if any, with the relevant licensing or regulatory authorities to help identify non-compliance with those laws and regulations that may have a material effect on the financial statements. Naturally, we remained alert to indications of (suspected) non-compliance throughout the audit. Finally, we obtained written representations that all known instances of (suspected) fraud or non-compliance with laws and regulations have been disclosed to us. Audit approach going concern We are responsible for obtaining reasonable assurance that the Company is able to continue as a going concern. Management is responsible to assess the Company’s ability to continue as a going concern and disclosing in the financial statements any events or circumstances that may cast significant doubt on the Company’s ability to continue as a going concern. As explained in note 2.1 ‘Statement of compliance and basis of preparation’ and note 26. ‘Financial risk management’ of the financial statements, management has prepared the financial statements of argenx SE based on the going concern assumption. No events or circumstances have been identified which cause significant doubt about the Company’s ability to continue its operations (going concern). Our procedures to evaluate the going concern assessment of management include: · Consider whether management’s assessment of going concern contains all relevant information of which we are aware as a result of our audit and review of the other information. In addition, we inquired with management about the key assumptions underlying the going concern assessment. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 356 |
| ·向管理層詢問他們對超出管理層評估期的事件和/或情況的瞭解。 ·我們將持續經營評估中使用的現金和現金等價物頭寸與2023年12月31日經審計的頭寸進行了核對。 ·我們評估了管理層自財務報表編制之日起至少12個月內準備的財務預測和分析。這包括: 通過評估歷史上實現的和未來預期的運營和資本支出來考慮關鍵基礎假設的合理性,以及評估評估的數學準確性。 ·我們評估了財務報表中關於持續經營的披露的充分性。 我們的審計程序沒有產生與管理層在應用持續經營假設時的 假設和判斷不符的結果。 我們的關鍵審計事項 關鍵審計事項是那些在我們的專業判斷中,在我們對財務報表的審計中具有最重要的意義。我們已將關鍵審計事項 通知董事會。關鍵審計事項並不是所討論的所有事項的綜合反映。 收入對淨額的總調整-請參閲財務報表的附註14和15 關鍵審計事項説明 公司確認與銷售VYVGART和 VYVGART HYTRULO產品有關的產品淨銷售額。這些產品淨銷售額根據IFRS 15與客户的合同收入(“IFRS 15”)入賬,據此,向客户銷售這些產品的確認金額反映了公司 預期有權換取這些商品的對價。產品總銷售額的大部分在美國,由於主要由對政府機構、分銷商、健康保險公司和受管理的醫療保健組織的返點組成的 可變對價的重要組成部分,這些產品的總銷售額可能會減少。如財務報表附註14和附註15所述,這些扣減項合計稱為毛額至淨額調整數。本公司確認的GTN調整是對未來期間將結清的相關債務的估計。 估計金額基於與醫療保健當局的合同安排、政府和州計劃以及總銷售額和第三方數據。 我們將美國產品淨銷售額的GTN調整確定為 關鍵審計事項,由於審計調整花費了大量精力,而且由於報告數據存在時間滯後,因此需要獲得足夠的適當審計證據來支持公司的估計。 審計中如何處理關鍵審計事項 我們與毛利率相關的審計程序包括以下內容: ·我們評估了關鍵收入合同和供應鏈合同,包括評估 根據IFRS 15對GTN調整的會計處理及其披露。 ·我們評估了服務提供商的獨立服務審計師報告,該報告由 公司用來代表公司處理毛利調整。 ·我們評估了公司制定GTN調整的方法和假設的適當性和一致性,包括測試管理層在其estimates. ar Gr g oup enx Factors Risk Go Corporate vernance資本中使用的基礎數據的完整性和準確性 股票財務 審查報表 財務非財務 信息 Argenx 2023年年度報告獨立審計師報告|357 |
| · We performed detailed testing procedures on a selection of adjustments by reconciling them to underlying evidence. · We performed recalculation procedures on the different components of management’s calculations. · We evaluated the Company’s ability to estimate the GtN adjustments by evaluating the historical accuracy of estimates made in the prior year in relation to the actuals incurred in this year. Observations The scope and nature of the audit procedures we performed was sufficient and appropriate to address the risks of material misstatement related to the GtN adjustments. Report on the other information included in The Annual Report The Annual Report contains other information, in addition to the financial statements and our auditor's report thereon. The other information consists of: · Management’s Report, including, amongst others, the Remuneration Report and Compensation Statement, and Non-Financial Information. · Other Information as required by Part 9 of Book 2 of the Dutch Civil Code. Based on the following procedures performed, we conclude that the other information: · Is consistent with the financial statements and does not contain material misstatements. · Contains all the information regarding the management report and the other information as required by Part 9 of Book 2 of the Dutch Civil Code. We have read the other information. Based on our knowledge and understanding obtained through our audit of the financial statements or otherwise, we have considered whether the other information contains material misstatements. By performing these procedures, we comply with the requirements of Part 9 of Book 2 of the Dutch Civil Code and the Dutch Standard 720. The scope of the procedures performed is substantially less than the scope of those performed in our audit of the financial statements. Management is responsible for the preparation of the other information, including Management’s Report in accordance with Part 9 of Book 2 of the Dutch Civil Code, and the other information as required by Part 9 of Book 2 of the Dutch Civil Code. Report on other legal and regulatory requirements and ESEF Engagement We were engaged by the Board of Directors as auditor of argenx SE on May 13, 2015, as of the audit for the year 2015 and have operated as statutory auditor ever since that financial year. No prohibited non-audit services We have not provided prohibited non-audit services as referred to in Article 5(1) of the EU Regulation on specific requirements regarding statutory audit of public-interest entities. European Single Electronic Format (ESEF) argenx SE has prepared its annual report in ESEF. The requirements for this are set out in the Delegated Regulation (EU) 2019/815 with regard to regulatory technical standards on the specification of a single electronic reporting format (hereinafter: the RTS on ESEF). ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 358 |
| 我們認為,以XHTML格式編制的年度報告,包括Argenx SE報告包中包含的(部分)標記的合併財務報表,在所有實質性方面都符合ESEF上的RTS。 管理層負責根據ESEF上的RTS編制包括財務報表的年度報告 ,因此,管理層將不同的組成部分合併到一個報告包中。 我們的責任是為我們的意見獲得合理的保證,以確定此報告包中的年度報告是否符合ESEF上的RTS。 我們按照荷蘭法律進行審查,包括荷蘭標準 3950N‘保證-opdrachten het voldoen aan de criteria voor het opstellen van en’(與遵守數字報告標準有關的保證活動)。 我們的檢查包括: ·瞭解公司的財務報告流程和標準包括編制報告包。 ·識別和評估年度報告在所有重大方面與ESEF上的RTS不符的風險,並針對這些風險設計和執行進一步的保證程序,為我們的意見提供依據,包括: ◦獲取報告包並執行驗證,以確定包含內聯XBRL實例和XBRL擴展分類的 報告包是否已按照ESEF上的 RTS中包含的技術規範編制; ◦檢查報告包中與合併財務報表相關的信息,以確定是否已應用所有要求的加價,以及 這些信息是否與ESEF上的RTS一致。 財務報表的責任説明 管理層和董事會對財務報表的責任 管理層負責根據歐盟-IFRS和荷蘭民法典第二冊第9部分編制和公平列報財務報表。 此外,管理層應負責進行內部控制,以確保財務報表的編制不會因欺詐或錯誤而出現重大錯誤。作為財務報表編制的一部分,管理層應負責評估公司持續經營的能力。根據所提到的財務報告框架,管理層應使用持續經營會計基礎編制財務報表,除非管理層打算清算公司或停止運營,或者別無選擇,只能這樣做。 管理層應在財務報表中披露可能對公司作為持續經營企業的持續經營能力產生重大懷疑的事件和情況。 董事會負責監督公司的財務reporting process. ar Gr g oup enx Factors Risk Go Corporate vernance資本 股票財務 審核報表 財務非財務 信息 argenx年度報告2023年獨立審計師報告|359 |
| Our responsibilities for the audit of the financial statements Our objective is to plan and perform the audit assignment in a manner that allows us to obtain sufficient and appropriate audit evidence for our opinion. Our audit has been performed with a high, but not absolute, level of assurance, which means we may not detect all material errors and fraud during our audit. Misstatements can arise from fraud or error and are considered material if, individually or in the aggregate, they could reasonably be expected to influence the economic decisions of users taken on the basis of these financial statements. The materiality affects the nature, timing and extent of our audit procedures and the evaluation of the effect of identified misstatements on our opinion. We have exercised professional judgement and have maintained professional scepticism throughout the audit, in accordance with Dutch Standards on Auditing, ethical requirements and independence requirements. Our audit included among others: · Identifying and assessing the risks of material misstatement of the financial statements, whether due to fraud or error, designing and performing audit procedures responsive to those risks, and obtaining audit evidence that is sufficient and appropriate to provide a basis for our opinion. The risk of not detecting a material misstatement resulting from fraud is higher than for one resulting from error, as fraud may involve collusion, forgery, intentional omissions, misrepresentations, or the override of internal control. · Obtaining an understanding of internal control relevant to the audit in order to design audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company's internal control. · Evaluating the appropriateness of accounting policies used and the reasonableness of accounting estimates and related disclosures made by management. · Concluding on the appropriateness of management's use of the going concern basis of accounting, and based on the audit evidence obtained, whether a material uncertainty exists related to events or conditions that may cast significant doubt on the company's ability to continue as a going concern. If we conclude that a material uncertainty exists, we are required to draw attention in our auditor's report to the related disclosures in the financial statements or, if such disclosures are inadequate, to modify our opinion. Our conclusions are based on the audit evidence obtained up to the date of our auditor's report. However, future events or conditions may cause the company to cease to continue as a going concern. · Evaluating the overall presentation, structure and content of the financial statements, including the disclosures. · Evaluating whether the financial statements represent the underlying transactions and events in a manner that achieves fair presentation. Because we are ultimately responsible for the opinion, we are also responsible for directing, supervising and performing the group audit. In this respect we have determined the nature and extent of the audit procedures to be carried out for group entities. Decisive were the size and/or the risk profile of the group entities or operations. On this basis, we selected group entities for which an audit or review had to be carried out on the complete set of financial information or specific items. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 360 |
| We communicate with the Board of Directors regarding, among other matters, the planned scope and timing of the audit and significant audit findings, including any significant findings in internal control that we identified during our audit. In this respect we also submit an additional report to the Board of Directors in accordance with Article 11 of the EU Regulation on specific requirements regarding statutory audit of public-interest entities. The information included in this additional report is consistent with our audit opinion in this auditor's report. We provide the Board of Directors with a statement that we have complied with relevant ethical requirements regarding independence, and to communicate with them all relationships and other matters that may reasonably be thought to bear on our independence, and where applicable, related safeguards. From the matters communicated with the Board of Directors, we determine the key audit matters: those matters that were of most significance in the audit of the financial statements. We describe these matters in our auditor's report unless law or regulation precludes public disclosure about the matter or when, in extremely rare circumstances, not communicating the matter is in the public interest. Rotterdam, March 21, 2024 Deloitte Accountants B.V. V.A.J. Fruytier ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Independent Auditor’s Report | 361 |
| 非財務 信息 7.1法規與合規363 7.2 NFRD 363 7.3歐盟分類學372 7 arginx 2023年度報告非財務信息|362 |
| 7 Non-Financial Information Regulations and Compliance The Company recognizes the importance of non-financial factors in creating long-term financial value, which involves identifying and mitigating aspects of economic activities that undermine non-financial value, as well as identifying and seizing opportunities to create the long-term value. We are dedicated to conducting our business in a safe and environmentally sustainable manner as part of our commitment to not only improve the lives of patients we hope to serve, but also to positively impact our stakeholders. The Company encourages the recently increased regulation in this area and does its utmost to comply with applicable regulations to the best of its ability. Currently, we are subject to the Directive 2014/95/EU of the European Parliament and of the Council of 22 October 2014 amending Directive 2013/34/EU as regards disclosure of non-financial and diversity information by certain large undertakings and groups (NFRD), as implemented in Dutch law and the Regulation (EU) 2020/852 of the European Parliament and of the Council of June 18, 2020 on the establishment of a framework to facilitate sustainable investment, and amending Regulation (EU) 2019/2088 (EU Taxonomy Regulation) and ancillary delegated regulations. For the financial year ending December 31, 2024, the Company will be subject to Directive (EU) 2022/2464 of the European Parliament and of the Council of December, 14 2022 amending Regulation (EU) No 537/2014, Directive 2004/109/EC, Directive 2006/43/EC and Directive 2013/ 34/EU, as regards corporate sustainability reporting (the CSRD), as well as the draft Dutch legislation which will become effective to implement same. In conjunction with its advisors, the Company commenced its preparations for CSRD reporting readiness during the course of mid-2023, including a detailed analysis to confirm the scope of additional disclosure which will be required to be made by the Company and updating the Company’s materiality assessment to bring in line with the double materiality approach contemplated by the CSRD (i.e., analyzing the impact of the Company’s business on society and the environment, but also analyzing how sustainability related matters affect the business of the Company). Reporting under the CSRD represents a significant increase to sustainability reporting when compared to the requirements of the NFRD. The Company will report under the CSRD for the first time in its next annual report. In this section, we make all disclosures required for our compliance with NFRD and the EU Taxonomy Regulation, and ancillary legislation and guidelines applicable to us. In addition to the non-financial disclosures made in this Annual Report, we have published a separate and dedicated report on ESG in 2023, of which an updated version will be published on or around the same date as this Annual Report, in which we give more context as well as additional, voluntary disclosures on ESG and related subjects. NFRD Introduction to the NFRD The NFRD requirements, applicable to argenx are included in Article 29a of Directive 2013/34/ EU (Accounting Directive). Article 29a of the Accounting Directive is implemented in Dutch law under Article 391 of Book 2 of the DCC in the Decree on the contents of the management report (Besluit inhoud bestuursverslag), in the Decree on the establishment of further provisions on the content of the annual report (Besluit tot vaststelling van nadere 7.1 7.2 7.2.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Regulations and Compliance | 363 |
| voorschriften omtrent de inhoud van het jaarverslag) and in the Decree on the publication of non-financial information (Besluit bekendmaking niet-financiële informatie). The NFRD requires ‘large companies’ to provide information on how they address and manage social and environmental challenges. In particular, companies are required to report on social, employee and environmental matters, human rights, bribery and anti-corruption, as well as board diversity in (consolidated) non-financial statements. To this end, the section below provides for the required disclosures. Report drafted pursuant to NFRD Business model We emerged from a break-through antibody engineering innovation and a philosophy that collaboration is key to success. Our business model revolves around our work to build an integrated, immunology company improving the lives of patients. As we continue to scale up the business to achieve this vision, it is critical that we do so with integrity and passion. To provide better, more effective products for patients, we also regularly engage healthcare professionals to provide various services in support of our business. The services provided by healthcare professionals include clinical investigations, advisory services, and speaking engagements at argenx events. We have built a reputation of consistent execution, hard work and integrity on our path to bring immunology breakthroughs to the patients who need them. Culture and behaviour When each of us acts with honesty and integrity, we gain the trust of our colleagues, patients and communities. We are dedicated to fostering a workplace where all people feel free to share their thoughts and ideas. All of our employees are therefore choosing to be part of a team looking to build the next great integrated immunology company that is rooted in science, data-based in our decision making and always focused on the patient. We have five cultural pillars that are at the core of argenx: · Innovation: our core mission is to innovate and do so at every step · Co-creation: we create through collaboration and we trust in the power of the team and know that together we are better · Empowerment: we share in our joint purpose and acknowledge that our people are our most valuable asset · Excellence: we have a quality culture and we want to do things right the first time and prioritize patient safety · Humility: this is the heart of our organization and we want to handle successes and challenges gracefully and learn from both Our Code of Conduct translates the core values into a set of clear standards to help guide our conduct as we navigate the complexities of the highly regulated and competitive global marketplace in which we operate as we work to become an independent, fully integrated, and global immunology company. Our commitment to the Code of Conduct enables our core business of innovation and our culture of collaboration. We are all dedicated to and responsible for its success. Each of us contributes to our reputation by living our core values every day and making the best choices for argenx and the many people we serve. Our cultural values create the work environment that has allowed us to deliver groundbreaking innovations to patients in the past, and is fundamental to sustain such an 7.2.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 364 |
| environment going forward. Consequently, our cultural values are essential for sustainable long term value creation within argenx. We have not identified any areas of our company culture that at this point require specific attention or change. When implementing this business model, we address and manage social and environmental challenges as described below. Social and employee matters Policies pursued, including due diligence processes We insist on building and maintaining a safe and secure work environment, where no one is subject to unnecessary risk. We commit to developing our people based on their strengths, to the benefit of the broader team. In this respect, we comply with international labor standards as well as applicable labor and employment laws, wherever we operate. This includes prohibiting child labor and forced labor, upholding the right to freedom of association, and eliminating discrimination at work. When selecting our business associates, we look for partners who share our values and our commitment to respecting and improving human rights and avoiding participating in forced, bonded or indentured labor or involuntary prison labor, the exploitation of children (including child labor), harsh or inhumane treatment or threat of any such treatment or any form of modern slavery or human trafficking. We believe open communication is critical to guaranteeing a positive work environment and our ultimate success. We understand that to make a difference we need to foster a culture of openness, where colleagues are encouraged to share their thoughts and ideas because diversity of thought leads to and empowers innovation. We actively listen to our colleagues and make sure all voices are heard. Our Code of Conduct sets out core principles for the way we commit to important employee and social matters, including our commitment to maintaining the highest scientific and ethical standards in our research and development activities and complying with all internationally accepted standards that apply to our clinical trials, including the ICH Guidelines for Good Clinical Practice and the ethical principles articulated in the Declaration of Helsinki, as well as applicable local laws and regulations. We monitor compliance with these standards through a number of policies which we regularly train relevant employees on. We operate a personal development program in which we encourage all employees to participate. We operate short-term and long-term incentive plans to encourage attraction and retention of qualified personnel. We take a stance against all forms of discrimination and commit to promoting diversity, equity and inclusion as set out in our Code of Conduct and in our diversity, equity and inclusion policy. We encourage respect of the individual, their integrity and their dignity, by ensuring that the working environment and relations between colleagues are free of discrimination and harassment, whether based on race, religion, color, political convictions, sex, language, pregnancy, ethnic or national origin, civil state, social status, sexual orientation, handicap, age or otherwise. We will protect any colleague who in good faith believes they are victims of harassment or discrimination. This includes actions that can reasonably be considered offensive, intimidating or discriminatory, including sexual harassment, power harassment and bullying, whether physical, verbal or visual. We encourage colleagues to speak up against any incident that could be viewed as harassment or discrimination and to support those affected. Once informed, we will take all measures required to stop any such behavior and to deal appropriately with the person or persons involved. The matter will be treated with discretion and diligence. We strictly prohibit retaliation or retribution against anyone who in good faith reports a concern about harassment, discrimination, or other issues, or cooperates with an 7.2.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 365 |
| investigation into alleged harassment and discrimination, even if the initial concern is ultimately determined to be unfounded, as is further set out in our Speak-up Policy. Outcome of those policies All employees have accepted and are trained (and retrained annually) on our Code of Conduct, and accepting, and committing to, the contents thereof is expected of all newcomers to argenx. At the date of this Annual Report, for the fiscal year ended December 31, 2023, we have not identified any material breaches of our Code of Conduct in relation to social or employee matters. Principal risks Our employees and relevant third parties may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements, which could have a material adverse effect on our business. Our future growth and ability to compete depends in part on our ability to retain key personnel and recruit additional qualified personnel. How these risks are managed In order to maintain oversight over compliance with the our Code of Conduct and other company policies including in relation to potential violations in the area of employee and social matters, and to increase compliance and ensure our colleagues know where to go with questions on the Code of Conduct and its application, we have established the argenx COMPASS Helpline, where our employees can raise any concerns they may have regarding potential violations of our policy confidentially or anonymously (to the extent allowed by law). We revised our Whistleblower Policy into our new Speak-up Policy to comply with Directive (EU) 2019/1937 of the European Parliament and of the Council of 23 October 2019 on the protection of persons who report breaches of EU law, which policies (jointly our Speak Up and Anti-retaliation Policy) enables and encourages our employees to speak up and report any suspected violation of our Code of Conduct, and to protect employees from retaliation. We have set-up a specific helpline reachable through different channels including by phone, also anonymously, to report any suspected potential violations. Also, to mitigate the risks of non-compliance with our Code of Conduct in relation to employee and social matters, we require all new employees to confirm their acceptance and adherence to the Code of Conduct and we train existing and new employees annually on our Code of Conduct and our Speak-up Policy. We offer competitive remuneration packages and share-based incentives in the form of an Equity Incentive Plan in which all employees are offered the opportunity to participate. We perform periodic benchmark analyses with an external service provider to ensure the competitiveness of the compensation offered to our key personnel in comparison to other (reference group) companies. We pay close attention to creating an environment that supports the further development of the talents of our key people, including through our personal development plan program. Non-financial key performance indicators At the date of this Annual Report, for the fiscal year ended December 31, 2023, we have not identified any material breaches of our Code of Conduct in relation to social or employee matters. Our voluntary employee turnover rate for fiscal year 2023 was 5% and our involuntary employee turnover rate for fiscal year 2023 was 2%, both numbers we believe to be below industry averages. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 366 |
| Environmental matters Policies pursued, including due diligence processes argenx is dedicated to conducting its business in a safe and environmentally sustainable manner as part of our commitment to not only improve the lives of patients we hope to serve, but also to positively impact our colleagues, business partners, and surrounding communities as well. In an effort to do this we: · comply with environmental laws and regulations that are related to our specific work and responsibilities. · encourage colleagues to respect the environment and natural resources available to us by taking sustainability steps like limiting energy use, reducing waste, and recycling. · have awareness and training programs to teach our employees how to deal with different waste systems. We are committed to expanding and developing our sustainability initiatives in the future. Given the present state of scientific knowledge, it is not possible to examine the complex interactions in a living organism solely by the use of modeling or performing experiments in cell cultures and tissue samples. Research using living animals remains essential in the discovery, development and production of new medicines. We cannot replace all animal experiments in the foreseeable future, but we continuously review the welfare and use of animals and develop procedures that reduce or replace animal experiments. If we engage in research using live animals, we follow all applicable laws and regulations, and argenx policies. We commit to treating research animals in a humane and responsible manner, in accordance with Code of Conduct and our Animal Welfare Policy. Our Animal Welfare Policy requires us to perform due diligence on third party collaborators who engage in research activities on our behalf, by reviewing their external certification on this topic (such as Association for Assessment and Accreditation of Laboratory Animal Care International certification) or if they have not (yet) been certified, by performing our own confirmatory due diligence through reviews and/or interviews or written questions and answers to gain comfort that the standards applied are at the same level as our internal standards on this topic. Compliance, audits and certification of all third parties is overseen by a management-level animal welfare committee, who are responsible for organizing regular lab visits in the EU. Where we are unable to perform in-person audits at our U.S.-based academic collaborators, or elsewhere, our audits are performed virtually. We do not currently have an environmental policy. We conduct our activities within the environmental regulatory framework set out by those jurisdictions in which we operate in and have obtained all required environmental licenses and permits. With the goal of mitigating the risk of failure to obtain any required environmental permits or licenses, or of losing granted permits or licenses we may need to operate our business, we regularly evaluate the requirements of such environmental permits and licenses to ensure continued compliance. Outcome of those policies All employees have accepted and are trained (and retrained annually) on our Code of Conduct, and accepting, and committing to, the contents thereof is expected of all newcomers to argenx. At the date of this Annual Report, for the fiscal year ended December 31, 2023, we have not identified any material breaches of our Code of Conduct in relation to environmental matters. Principal risks We have assessed our activities to date and did not identify specific risks of material environmental violations and as such we have not identified environmental risks as principal risks for argenx. Our primary research and development activities take place in our facilities in 7.2.4 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 367 |
| Zwijnaarde, Belgium. For these activities we require, and have obtained, the necessary environmental and biohazard permits from the responsible governments, required by us for the manner in which we use said facilities. We may become exposed to liability and substantial expenses in connection with environmental compliance or remediation activities. Our personnel could breach the animal welfare commitments set out in our Code of Conduct or our Animal Welfare Policy. How these risks are managed We comply with environmental laws and regulations that are related to our specific work and responsibilities and offer training to our employees depending on their area of work. In addition, we have a dedicated safety advisor and facility manager supervising compliance with environmental law on our premises. All employees receive health and safety training relevant to their specific job role. We train all personnel involved in research activities with live animals, on our Animal Welfare Policy. The COMPASS Helpline enables us to maintain oversight over compliance with our Code of Conduct and other Company policies including in relation to potential violations in the area of environmental matters, and to increase compliance and ensure our colleagues know where to go with questions on the Code of Conduct and its application. Employees can raise any concerns they may have regarding potential violations of our Code of Conduct confidentially or anonymously (to the extent allowed by law) through our COMPASS Helpline, including in relation to violations of our Code of Conduct on environmental matters or in relation to violations of our Animal Welfare Policy. Non-financial key performance indicators At the date of this Annual Report, for the fiscal year ended December 31, 2023, we have not identified any material breaches of our Code of Conduct in relation to environmental matters, and we have not identified any material breaches of our Animal Welfare Policy. Matters with respect to human rights Policies pursued, including due diligence processes We commit to compliance with international labor standards as well as applicable labor and employment laws, wherever we operate. This includes prohibiting child labor and forced labor, upholding the right to freedom of association, and eliminating discrimination at work. When selecting our business associates, we strive to work with third parties who share our commitment to respecting and improving human rights, and we do not conduct business with any individual or company that participates in forced, bonded or indentured labor or involuntary prison labor, the exploitation of children (including child labor), harsh or inhumane treatment or threat of any such treatment or any form of modern slavery or human trafficking. Our Code of Conduct includes our commitment to respecting the human rights of all people and ensure fairness in the workspace. All our personnel is trained annually on our Code of Conduct including its provisions on respecting human rights. Accepting, and committing to, the contents of the aforementioned Code of Conduct is expected of all newcomers to argenx. Outcome of those policies In fiscal year 2023, there were no alleged breaches of our Code of Conduct on the topics of human rights or alleged forced labor or child labor. Principal risks We have assessed our activities to date and did not identify specific risks of violations of human rights in relation to our business activities and as such we have not identified the risk of violations of human rights as principal risk for argenx. 7.2.5 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 368 |
| 如何管理這些風險 為了保持對我們的行為準則和其他公司政策的遵守情況的監督,包括與人權領域的潛在違反行為有關的遵守情況,並 為了提高合規性並確保我們的同事知道如何處理有關行為準則及其應用的問題,我們建立了Argenx Compass幫助熱線, 我們的員工可以在這裏祕密或匿名(在法律允許的範圍內)提出他們對可能違反我們政策的任何擔憂,包括與違反我們的人權相關主題的行為準則有關的問題。 非金融關鍵績效指標 在2023財年,在人權或所謂的強迫勞動或童工問題上,沒有違反我們的行為準則的指控。 與反腐敗和 所奉行的賄賂政策有關的問題,包括盡職調查程序 我們不容忍賄賂或腐敗行為,無論是在我們的直接業務交易中,還是由代表我們行事的 第三方。我們從不提供、承諾或提供任何有價值的東西給 不正當地影響業務決策或為了獲得或保留業務的目的。 我們的行為準則和我們的全球反賄賂和腐敗政策適用於我們的 員工以及代表Argenx工作的顧問(包括臨時承包商), 規定了適用於我們運營的每個市場中的每個互動的以下最低標準-即使這些標準高於其他市場的標準,以及 即使這可能意味着放棄某些商業機會: ·遵守當地和國際法律法規。我們致力於 遵守所有涉及賄賂和腐敗的國際和當地法律法規。因此,我們的員工和代表我們 行事的人員有責任瞭解並遵守適用於他們的工作角色和他們開展業務的國家/地區的法律法規。這在賄賂和腐敗領域尤為重要 ,許多國家已經實施了 法律,如《反海外腐敗法》和《英國反賄賂法》,這些法律的範圍都是國際的。 ·避免不適當的影響。我們致力於開展不受腐敗影響的業務。任何員工或代表我們工作的任何人都不應直接或通過中介向任何人提供或提供任何有價值的東西,以獲得 不正當的商業優勢,代表Argenx的任何人也不應接受第三方的任何有價值的東西以換取優惠待遇。 ·第三方。我們在與業務第三方(包括分銷商、供應商或代表我們運營的其他人)達成安排時要謹慎行事。作為一般規則,我們承諾絕不通過業務中介機構做任何我們自己不允許做的事情。 此外,當我們聘請醫療保健專業人員或政府官員作為 顧問、顧問、調查員、演講者或任何其他身份時,我們要求滿足以下 要求: ·必須有書面證明的合法業務需求 argenx部分服務。不得建立業務關係作為誘使或獎勵醫療保健專業人員或政府官員開出、購買或推薦Argenx產品的變相手段。 7.2.6 ar Gr g oup enx Factors Risk Go Corporate vernance資本 共享財務 審核報表 財務非財務 信息 Argenx 2023年NFRD年度報告|369 |
| ·醫療保健專業人員或政府官員的選擇必須基於他們的資歷、專業知識、能力、與確定的需求直接相關的經驗和其他適當標準。 ·必須在服務開始之前簽署書面合同,以準確描述服務的性質和薪酬基礎。 ·醫療保健專業人員或政府官員的所有薪酬必須反映所提供服務的公平 市場價值。 ·由Argenx組織或贊助的涉及醫療保健專業人員或政府官員服務的會議或活動必須在有利於會議或活動的 目的的適當場所舉行。 旅行的所有安排(或費用報銷),向醫療保健專業人員或政府官員提供的與其服務績效相關的住宿和餐飲必須符合公司政策。我們確保通過避免贈送禮物或其他貴重物品來避免被認為存在不正當影響。 這些政策的結果 在2023財年,我們沒有發現在反腐敗或反賄賂問題上違反了我們的行為準則或全球反賄賂和腐敗政策。 主要風險 我們可能受到醫療保健法律、法規和執法的約束。我們不遵守這些法律可能會損害我們的運營結果和財務狀況。由於我們的許多醫療保健專業人員也是我們的客户,因此患者和其他人 可能會察覺到潛在的利益衝突,即使根本不存在。未能遵守適用的醫療保健法律法規可能會導致強制執行,包括民事和 行政處罰、罰款或刑事起訴,並可能導致我們產生巨大的 成本並損害我們的業務和聲譽。 如何管理這些風險 為了避免甚至出現利益衝突的跡象,我們以最高誠信與醫療保健專業人員進行所有互動,嚴格遵守政府和 行業機構的法規,並執行我們自己的嚴格內部指導方針。我們已 設計並實施了一套有針對性的合規計劃,其中包括一系列規範、政策和程序,我們積極定期對所有相關人員進行培訓。我們 招募了一支專門的法律和合規團隊來支持和監控相關規章制度的合規性 。此外,我們每年都會對所有員工進行行為準則培訓,包括關於反賄賂和反腐敗的規定。所有Argenx的新來者都應該接受並承諾其中的內容。我們建立了Argenx Compass幫助熱線,以保持對我們的行為準則和其他公司政策的遵守情況的監督,包括與 反賄賂和反腐敗領域的潛在違規行為有關的監督,並提高合規性並確保我們的 同事知道如何處理有關行為準則及其應用的問題。 員工可以祕密或匿名(在法律允許的範圍內)提出他們對可能違反我們政策的任何擔憂,包括違反我們人權相關主題的《行為準則》。 2023財年非金融關鍵績效指標 ,我們沒有發現在反腐敗或反賄賂方面違反我們的《行為準則》或我們的全球反賄賂和腐敗政策 matters. ar Gr g oup enx Factors Risk Go Corporate vernance資本共享財務 審查聲明 財務非財務 信息 Argenx 2023年年度報告|370 |
| Insight into our diversity, equity and inclusion policy and practices Policies pursued, including due diligence processes We value diversity among our colleagues as an integral component in building a sustainable growth platform. We believe that a diverse workforce enhances our overall performance and success. We take pride in creating and sustaining a culture and environment where each of us can excel. We bring together people with diverse backgrounds experiences and functional expertise. By doing so, we broaden the scope of ideas and creativity essential to developing and delivering innovative therapies to patients. Acknowledging and benefiting from different perspectives promotes diversity of thought and empowers innovation. It also contributes to our commitment to improve lives of patients, wherefore we need teams with a healthy mix of contrasting perspectives and backgrounds that reflect the diverse communities we serve. We recognize that our people are our greatest strength. Fostering an inclusive work environment where everyone feels safe and encouraged to contribute leads to better work outcomes and supports high levels of employee commitment and retention. We aspire to be a consciously global company. Our success is built on, and dependent on true collaboration in cross-functional and often cross-regional teams in which open communication is encouraged and safeguarded. Everyone has a voice and is encouraged to contribute to the benefit of our common goals, irrespective of race, ethnicity, age, gender or cultural background. Good ideas as well as real concerns are taken seriously, regardless of who brings them forward. How our diversity, equity and inclusion policy is being implemented. Our diversity, equity and inclusion policy is implemented in the way we recruit, develop and promote our employees and Board of Directors. We value our fair, inclusive recruitment process, which is standardized across the organization and focuses on pre-identified ‘what counts’ factors. The process involves a diverse group of colleagues from across the organization, who are provided with training to recognize any existing biases. Recruitment decisions are based on a group evaluation of available candidates, ensuring different perspectives. Our onboarding program is designed to promote inclusion by building a strong social fabric across teams, functions and geographic locations. Furthermore, all employees are encouraged to participate in a personal development program aimed at building on their individuals strengths to benefit the broader team. We offer opportunities for promotion, training and career development solely based on job-related, appropriate criteria such as skills, competencies, experience, aptitude and enthusiasm and giving account to each individual’s experience, ambitions and capabilities. We will continue to implement our diversity, equity and inclusion policy by seeking new ways to improve and support diversity, equity and inclusion in our company. Diversity targets We aim to foster an inclusive work environment in support of our strategic plan and priorities. We continue to raise the bar in this regard, and to commit to measures and goals designed to support our maturing company culture. We aim to have an equal gender balance in our Board of Directors and in our Company leadership (including functional leaders and project leaders). The outcome of those policies, results of the Diversity, Equity and Inclusion Policy As at December 31, 2023, our Board of Directors consisted of nine directors, including one executive director and eight non-executive directors. Of the directors who chose to disclose their gender, the Board of Directors contained five male directors and three female directors (non-executive directors), translating into a 55.55% male/331/3% female balance for our full Board of Directors (compared to five males and three females (non-executive directors) (55.55%/331/3%) as of December 31, 2022) and a 62.5% male/37.5% female balance for our non-executive directors (compared to 62.5% male/37.5% female as of December 31, 2022). As 7.2.7 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 NFRD | 371 |
| at December 31, 2023 and December 31, 2022, our Company leadership team consisted of 31 persons, comprised of a mix of 19 males and 12 females, (61%/39% respectively). Our leadership consists of all full time employees reporting directly to our CEO, as well as all (other) leaders of our largest functions and projects. Each of these positions is characterized by a high impact across the organization, leading a global and cross functional team and having a global reach. We estimate that as of December 31, 2023, 58% of our workforce were female and 42% were male (compared to 63% female and 37% male as of December 31, 2022). EU Taxonomy Introduction to the EU Taxonomy Regulation The EU Taxonomy Regulation entered into force on July 12, 2020 and establishes the general framework for determining whether an economic activity qualifies as environmentally sustainable for the purposes of establishing the degree to which an investment is environmentally sustainable. The EU taxonomy framework will develop over time. Article 9 of the EU Taxonomy Regulation identifies six environmental objectives: (1) climate change mitigation; (2) climate change adaptation; (3) the sustainable use and protection of water and marine resources; (4) the transition to a circular economy (5) pollution prevention and control; and (6) the protection and restoration of biodiversity and ecosystems. The EU Commission has adopted a catalogue of economic activities that can be taken into account for these objectives. On April 21, 2021, the EU Commission adopted the Commission Delegated Regulation (EU) 2021/2139 of June 4, 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by establishing the technical screening criteria for determining the conditions under which an economic activity qualifies as contributing substantially to climate change mitigation or climate change adaptation and for determining whether that economic activity causes no significant harm to any of the other environmental objectives (the Climate Delegated Act), which became effective in January 2022. The EU Commission further adopted the Commission Delegated Regulation (EU) 2021/2178 of July 6, 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by specifying the content and presentation of information to be disclosed by undertakings subject to Articles 19a or 29a of Directive 2013/34/EU concerning environmentally sustainable economic activities, and specifying the methodology to comply with that disclosure obligation (the Article 8 CDR), which also became effective in January 2022. On March 9, 2022, the EU Commission adopted a complementary climate delegated act including, under strict conditions, specific nuclear and gas energy activities in the list of economic activities covered by the EU taxonomy. It was published in the Official Journal on July 15, 2022 and became effective in January 2023. 7.3 7.3.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 372 |
| On June, 27 2023, the Commission adopted final delegated acts, supporting the EU Taxonomy Regulation. This includes Delegated Regulation (EU) 2023/2486 of June 27, 2023 (the Environmental Delegated Act). These delegated acts updates to the mandatory reporting templates, set out technical screening criteria for additional activities for the first two environmental objectives; climate change adaptation and climate change mitigation. These delegated acts also introduce technical screening criteria and relating reporting obligations for activities pursuing the remaining four environmental objectives; (i) sustainable use and protection of water and marine resources, (ii) transition to a circular economy, (iii) pollution prevention and control, and (iv) protection and restoration of biodiversity and ecosystems (together with the first two environmental objectives, hereinafter referred to as the Taxonomy Environmental Objectives). These delegated acts were published in the Official Journal on November, 21 2023 and apply as of January 1, 2024 for annual reporting periods occurring in 2023. As such, these requirements have been taken into account in this annual report. In this section we present our compliance with the EU Taxonomy Regulation, the Climate Delegated Act, the Environmental Delegated Act, the Article 8 CDR and ancillary legislation currently applicable to us (the EU Taxonomy Legal Framework). Compliance with the EU Taxonomy Regulation In 2023 we performed a reassessment of all potential taxonomy-eligible economic activities, including all Taxonomy Environmental Objectives, listed in the Climate Delegated Act and the Environmental Delegated Act, based on our activities as a biopharmaceutical group for the current year’s activity. The Climate Delegated Act focuses on those economic activities and sectors that have the greatest potential to achieve the Taxonomy Environmental Objectives. The sectors covered include energy, selected manufacturing activities, transport and buildings. Our assessment methodology for 2023 is listed below and is based on the EU Taxonomy Legal Framework applicable as of January 1, 2024. Companies are required to identify if their activities are eligible under the EU Taxonomy Regulation. On the basis of Commission Delegated Regulation (EU) 2023/137 amending Regulation (EC) No 1893/2006 of the European Parliament and of the Council establishing the statistical classification of economic activities NACE Revision 2, our main activities are: • NACE 72.10 – Research and experimental development on natural sciences and engineering. According to the Dutch national transposition of the NACE code, the more specific level of the NACE code for our main activity is NACE 72.11 – Research and experimental development on biotechnology; and • NACE 46.46 – Wholesale of pharmaceutical and medical goods. Our assessment of taxonomy-eligibility is focused on economic activities, defined as the provision of goods or services on a market, thus (potentially) generating revenues. In this context, we, as a commercial-stage biopharmaceutical group, define the research and development and marketing of pharmaceutical products and wholesale thereof as the core of our business activities. We define activities such as the manufacturing or the transport of our pharmaceutical products to our clients as underlying activities necessary to conduct our core business activities. All of the activities mentioned in this paragraph will hereinafter be referred to as the argenx Activities. Following a thorough analysis of the EU Taxonomy Legal Framework, we do not consider the argenx Activities to be in scope of the Climate Delegated Act or the Environmental Delegated Act. We have concluded that the argenx Activities qualify as EU Taxonomy non-eligible 7.3.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 373 |
| economic activities and that they do not substantially contribute to any of the Taxonomy Environmental Objectives. Future EU Taxonomy disclosures We are committed to the continued and ongoing assessment of our taxonomy eligibility on an annual basis and note that the required disclosures under the EU Taxonomy Legal Framework will keep evolving. Turnover Eligibility and Alignment Since the argenx Activities are EU Taxonomy non-eligible activities, they are not included in our turnover key performance indicators (KPI). We have concluded that as eligibility for the argenx Activities is nil, alignment related to turnover is also considered to be nil and totals 0%. Our net turnover KPI denominator, covering product net sales and collaboration revenue (as listed in Annex I, point 1.1.1 of Article 8 CDR), totals $1.2 billion. Non-financial undertakings related to turnover are included in the consolidated financial statements, under footnote 15, Product net sales and footnote 16, Collaboration revenue. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 374 |
| Proportion of turnover from products or services associated with Taxonomy-aligned economic activities – disclosure covering 2023 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) Economic activities Code(s) Turnover Proportion of Turnover Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Mini-mum safe-guards Propor-tion of taxo-nomy-aligned (A.1.) or eligible (A.2.) Turnover, year 2022 Cate-gory (ena-bling activity) Cate-gory (transi-tional activity) A. TAXONOMY-ELIGIBLE ACTIVITIES A.1 Environmentally sustainable activities (Taxonomy-aligned) EUR % Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL % Y; N; N/EL Y; N; N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL Turnover of environmentally sustainable activities (Taxonomy-aligned) (A.1) – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 Of which Enabling – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL Of which Transitional – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 375 |
| 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) A.2 Taxonomy-eligible but not environmentally sustainable activities (Taxonomy-non-aligned activities) EUR % EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL % – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 Turnover of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) – 7,135 18 0 Total (A.1 + A.2) – 7,204 18 0 B. TAXONOMY-NON-ELIGIBLE ACTIVITIES EUR % Turnover of Taxonomy-non-eligible activities (B) 1,226,316 100 Total (A + B) 1,226,316 100 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 376 |
| CapEx Eligibility and Alignment We have concluded that as eligibility for the argenx Activities is nil, alignment related to CapEx is also considered to be nil and totals 0%. Our denominator for calculation of CapEx KPIs, covering tangibles and intangible assets during the financial year (as listed in Annex I, point 1.1.2.1 of Article 8 CDR) totals $68 million. Non-financial undertakings related to CapEx are listed in the consolidated financial statements, included as additions in footnote 4, Property, plant and equipment and footnote 5, Intangible assets. Proportion of CapEx from products or services associated with Taxonomy-aligned economic activities – disclosure covering 2023 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) Economic activities Code(s) CapEx Propor-tion of CapEx Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Mini-mum safe-guards Propor-tion of taxo-nomy-aligned (A.1.) or eligible (A.2.) CapEx, year 2022 Cate-gory (ena-bling activity) Cate-gory (transi-tional activity) A. TAXONOMY-ELIGIBLE ACTIVITIES A.1 Environmentally sustainable activities (Taxonomy-aligned) EUR % Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL % Y; N; N/EL Y; N; N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL CapEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 Of which Enabling – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL Of which Transitional – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 377 |
| 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) A.2 Taxonomy-eligible but not environmentally sustainable activities (Taxonomy-non-aligned activities) EUR % EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL % – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 CapEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) – 7,135 18 0 Total (A.1 + A.2) – 7,204 18 0 B. TAXONOMY-NON-ELIGIBLE ACTIVITIES EUR % CapEx of Taxonomy-non-eligible activities (B) 147,903 100 Total (A + B) 147,903 100 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 378 |
| OpEx Eligibility and Alignment We have concluded that as eligibility for the argenx Activities is nil, alignment related to OpEx is also considered to be nil and totals 0%. Our denominator for calculation of OpEx KPIs, covering non-capitalized costs such as research and development, building renovation, short-term lease, maintenance and repair, and day to day service of plant, property and equipment during the financial year (as listed in Annex I, point 1.1.3.1 of Article 8 CDR), totals $859.5 million across research and development only, as the remaining topics defined are not currently part of operational expenditure. Research and development only undertakings related to OpEx are listed in the consolidated financial statements, included in footnote 19, Research & development expense. Proportion of OpEx from products or services associated with Taxonomy-aligned economic activities – disclosure covering 2023 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) Economic activities Code(s) OpEx Propor-tion of OpEx Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Climate change miti-gation Climate change adap-tation Water Pollu-tion Circular eco-nomy Biodi-versity Mini-mum safe-guards Propor-tion of taxo-nomy-aligned (A.1.) or eligible (A.2.) OpEx, year 2022 Cate-gory (ena-bling activity) Cate-gory (transi-tional activity) A. TAXONOMY-ELIGIBLE ACTIVITIES A.1 Environmentally sustainable activities (Taxonomy-aligned) EUR % Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL Y; N; N/EL % Y; N; N/EL Y; N; N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL – – – 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL N/EL OpEx of environmentally sustainable activities (Taxonomy-aligned) (A.1) – 0.00 0 N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 Of which Enabling – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL Of which Transitional – – – N/EL N/EL N/EL N/EL N/EL N/EL N/EL 0 N/EL ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 379 |
| 2023 Substantial contribution criteria DNSH criteria (Do no significant harm) A.2 Taxonomy-eligible but not environmentally sustainable activities (Taxonomy-non-aligned activities) EUR % EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL EL; N/EL % – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 – – – 0 EL N/EL N/EL N/EL N/EL N/EL 0 OpEx of Taxonomy-eligible but not environmentally sustainable activities (not Taxonomy-aligned activities) (A.2) – – 0 0 – 0 Total (A.1 + A.2) – – 0 0 B. TAXONOMY-NON-ELIGIBLE ACTIVITIES EUR % OpEx of Taxonomy-non-eligible activities (B) 479,934 100 Total (A + B) 479,934 100 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 EU Taxonomy | 380 |
| Glossary 8.1 Cross Reference table for annual reporting requirement 382 8.2 Management Confirmations 383 8.3 Definitions 384 8 argenx Annual Report 2023 Glossary | 381 |
| 8 Glossary Cross Reference table for annual reporting requirement The following list of cross references identifies where each item required for us to disclose in our yearly financial report can be found in this Annual Report. Source of Requirement Topic Location Article 2:391 DCC, RJ 400, RJ 405 Report on the Company’s activities Shareholder Letter Presentation of the Group Corporate structure General Description of the Company and its Share Capital Board of Directors report Corporate Governance Primary risks and uncertainties Risk Factors Risk appetite & control Risk Appetite & Control Analysis of financial condition and results Operating and Financial Review Information on research and development activities Our Products and Product Candidates Collaborations and Licenses Forward looking paragraph 2024 Outlook Compensation statements and remuneration report Remuneration Report and Compensation Statement RJ 430 Key figures, ratios etc. Operating and Financial Review Article 2:392 DCC/RJ 410 Auditor’s opinion Attached to the 2023 Annual Report included herein Articles of association on the distribution of profits Articles of Association on Profits, distributions and losses List of subsidiaries Company Profile – Group Structure Decree on contents of board report (Besluit inhoud bestuursverslag), Article 2:391 sub 5 DCC Corporate governance code comply-or-explain Dutch Corporate Governance Code, “Comply or Explain” Main elements of financial management & control systems in connection with the company’s financial reporting Financial Risks and Controls Functioning of the general meeting General Meeting and Voting Rights Composition and functioning of the board of directors and its committees Board of Directors Non-Executive Directors Article 10 Decree Takeover Directive (Besluit artikel 10 overnamerichtlijn), Capital structure General description of the Company and its Share Capital 8.1 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Cross Reference table for annual reporting requirement | 382 |
| Source of Requirement Topic Location Article 2:391 sub 5 DCC Principal shareholders Share Classes and Principal Shareholders Particular shareholder rights and limitations thereof General Meetings and Voting Rights Procedure for appointment of board members Management Structure Procedure for amending the articles of association Amendment of Articles of Association Authority of the board of directors to issue or acquire shares Issue of Shares Acquisition of Shares in our Capital Material arrangements, to which the company is a party, in relation to a public offer Anti-Takeover Provisions RJ = Guidelines on Annual Reporting (Richtlijnen voor de Jaarverslaggeving) Management Confirmations With due regard to best practice provision 1.4.3 of the DCGC, we confirm that: i. This Annual Report provides sufficient insights into any failings in the effectiveness of the internal risk management and control systems, with regard to the risks as referred to in best practice provision 1.2.1 of the DCGC, as is further substantiated in section 2 “Risk Factors,” and section 3 “Corporate Governance”. ii. The risk- and control systems described herein, particularly in paragraph 3.9.5 “Financial Risks and Controls” provide reasonable assurance that the financial reporting does not contain any material inaccuracies; iii. We confirm that we expect that our existing cash and cash equivalents and current financial assets will enable us to fund our operating expenses and capital expenditure requirements through at least the next 12 months. On the basis of the current state of affairs, it is justified that the financial reporting is prepared on a going concern basis; and iv. This Annual Report, particularly section 2 “Risk Factors” states the material risks, as referred to in best practice provision 1.2.1 and the uncertainties, to the extent that they are relevant to the expectation of our continuity for the period of 12 months after the preparation of this Annual Report. The aforementioned statement does not in any way limit the relevance or applicability of the Risk Factors set out in this Annual Report to the aforementioned period of 12 months. Signed on behalf of argenx SE 8.2 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Management Confirmations | 383 |
| Definitions The following explanations are intended to assist the general reader to understand certain terms used in this Annual Report. The definitions set out below apply throughout this Annual Report, unless the context requires otherwise. Term Definition 2021 Remuneration Policy 2021 remuneration policy 2023 20-F Form 20-F for the year ended December 31, 2023 2023 General Meeting annual General Meeting was held on May 2, 2023 2024 General Meeting the Company’s annual General Meeting that will take place on May 7, 2024 AAV ANCA-associated vasculitis AbbVie AbbVie, Inc. AbbVie Collaboration Agreement the collaboration agreement with AbbVie, Inc. to develop and commercialize ARGX-115 (ABBV-151) as a cancer immunotherapy against the novel target GARP ACA the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act of 2010 Accounting Directive Directive 2013/34/EU AChR anti-acetylcholine receptor AChR-AB+ AChR antibody positive ADCC antibody-dependent cell-mediated cytotoxicity ADSs American depositary share AFM the Dutch Authority for the Financial Markets (Stichting Autoriteit Financiële Markten) Agomab Agomab Therapeutics NV AKS the U.S. federal Anti-Kickback Statute Alexion Alexion Pharmaceuticals, Inc. ALS amyotrophic lateral sclerosis Amgen Amgen, Inc. AML acute myeloid leukemia AMP average manufacturer price AMR antibody-mediated rejection Annual Report this annual report argenx Activities the argenx activities identified as core activities for the purposes 8.3 ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 384 |
| of the EU Taxonomy Legal Framework, such activities being research and development and marketing of pharmaceutical products and wholesale thereof argenx or the Company argenx SE Article 8 CDR Commission Delegated Regulation (EU) 2021/2178 of July 6, 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by specifying the content and presentation of information to be disclosed by undertakings subject to Articles 19a or 29a of Directive 2013/34/EU concerning environmentally sustainable economic activities, and specifying the methodology to comply with that disclosure obligation Articles of Association our current articles of association Asset Development Agreement the asset development agreement entered into with IQVIA ASyS anti-synthetase syndrome AV anti-neutrophil cytoplasmic antibody-associated Vasculitis B-cell B-lymphocyte BioWa BioWa, Inc BioWa Agreement non-exclusive license agreement entered into with BioWa BLA biologics license application Board By-Laws the rules adopted by our Board of Directors that describe the procedure for holding meetings of the Board of Directors, for the decision-making by the Board of Directors and the Board of Directors’ operating procedures Board of Directors consisting of our executive director(s) and our non-executive directors. BP bullous pemphigoid BPCIA the U.S. Biologics Price Competition and Innovation Act Broteio Broteio Pharma B.V. Broteio Agreement collaboration agreement entered into with Broteio C2 component 2 CapEx capital expenditure CBA collective bargaining agreement CCPA California Consumer Privacy Act of 2018 CEO chief executive officer CFO chief financial officer cGMPs current good manufacturing practices CHMP Committee for Medicinal Products for Human Use ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 385 |
| Chugai Chugai Pharmaceutical Co., Ltd. CIDP chronic inflammatory demyelinating polyneuropathy Climate Delegated Act Commission Delegated Regulation (EU) 2021/2139 of June, 4 2021 supplementing Regulation (EU) 2020/852 of the European Parliament and of the Council by establishing the technical screening criteria for determining the conditions under which an economic activity qualifies as contributing substantially to climate change mitigation or climate change adaptation and for determining whether that economic activity causes no significant harm to any of the other environmental objectives CMOs contract manufacturing organizations CMS Congenital myasthenic syndrome or Centers for Medicare & Medicaid, as the context dictates Code of Conduct our Code of Business Conduct and Ethics COMP European Medicines Authority’s Committee for Orphan Medicinal Products Concerned Member States the competent authorities of all European Union Member States in which an application for authorization of a clinical trial has been submitted COO chief operating officer CPRA California Privacy Rights Act of 2020 CRmin minimal dose of steroids CRO contract research organization CRR complete renal response CSRD Directive (EU) 2022/2464 of the European Parliament and of the Council of December, 14 2022 amending Regulation (EU) No 537/ 2014, Directive 2004/109/EC, Directive 2006/43/EC and Directive 2013/34/EU, as regards corporate sustainability reporting CTA clinical trial application CTR EU Regulation No 536/2014 of the European Parliament and of the Council of April, 16 2014 on clinical trials on medicinal products for human use, and repealing Directive 2001/20/EC (clinical trials regulation) DCC Dutch Civil Code (Burgerlijk Wetboek) DCGC the Dutch Corporate Governance Code 2022 Deloitte Deloitte Accountants B.V. DFSA Dutch Financial Supervision Act (Wet op het financieel toezicht) DGF delayed graft function DHS dehydrated hereditary stomatocytosis ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 386 |
| Dividend Received Deduction deduction of 100% of the gross dividend received from taxable income DM dermatomyositis DPO data protection officer Draft 2024 Remuneration Policy the Company’s draft 2024 remuneration policy, which expected to be published in draft form on or around March 21, 2024 DSA donor specific antibodies ECDRP European Commission Decision Reliance Procedure ECL expected credit loss EEA European Economic Area Elektrofi Elektrofi, Inc. Elektrofi Agreement collaboration and license agreement entered into with Elektrofi EMA European Medicines Authority ENHANZE® ENHANZE technology ENHANZE® License Agreement in-license agreement entered into with Halozyme, Inc. Enterprise Chamber the Dutch Enterprise Chamber of the Amsterdam Court of Appeal (Ondernemingskamer van het Gerechtshof te Amsterdam) Environmental Delegated Act Delegated Regulation (EU) 2023/2486 of June 27, 2023 e-Privacy Directive Directive 2002/58/EC of the European Parliament and of the Council of July 12, 2002 Equity Incentive Plan the equity incentive plan as adopted by our Board of Directors on December 18, 2014, which was approved by the General Meeting on May 13, 2015, and amended by the General Meeting on April 28, 2016, and November 25, 2019, and the Board of Directors on December 18, 2019, November 5, 2020, December 15, 2021 and on February 27, 2023 ESG environmental, social and corporate governance ETASU elements to assure safe use EU European Union EU-IFRS International Financial Reporting Standards and the interpretations issued by the IASB’s International Financial Reporting Interpretation Committee as adopted by the European Union Euronext Brussels the regulated market operated by Euronext Brussels SA/NV, a regulated market within the meaning of Directive 2014/65/EU of the European Parliament and of the Council of May 15, 2014, on markets in financial instruments amending Council Directives 2004/39/EC, Directive 85/611/EEC, 93/6/EEC and Directive 2000/ ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 387 |
| 12/EC of the European Parliament and of the Council and repealing Council Directive 93/22/EEC (MiFID II) EU Taxonomy Legal Framework the EU Taxonomy Regulation, the Climate Delegated Act, the Environmental Delegated Act, the Article 8 CDR and ancillary legislation currently applicable to us EU Taxonomy Regulation Regulation (EU) 2020/852 of the European Parliament and of the Council of June 18, 2020 on the establishment of a framework to facilitate sustainable investment, and amending Regulation (EU) 2019/2088 Exchange Act the U.S. Securities Exchange Act of 1934, as amended Fc antibody region interacting with cell surface Fc receptors FCP federal ceiling price FcRn neonatal Fc receptor FDA U.S. Food and Drug Administration FDCA the U.S. Federal Food, Drug, and Cosmetic Act FDORA Food and Drug Omnibus Reform Act FSS federal supply schedule FTT Financial Transaction Tax Fujifilm FUJIFILM Diosynth Biotechnologies Denmark ApS FVTOCI fair value through other comprehensive income FVTPL fair value through profit or loss GARP glycoprotein A repetitions predominant GARP Agreement a collaboration and exclusive product license agreement with UCL and its technology transfer company Sopartec GARP License exclusive, worldwide commercial in-license for use of certain GARP-related intellectual property rights owned by UCL and the Ludwig Institute for Cancer Research GCC Gulf Cooperation Council, comprising Saudi Arabia, Kuwait, the United Arab Emirates, Qatar, Bahrain and Oman GCPs good clinical practices GDPR Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data General Meeting any general meeting of shareholders of argenx SE (i.e., any annual general meeting and any extraordinary general meeting) Genmab Genmab A/S Genpharm Genpharm Services FZ-LLC Genpharm Agreement partnership agreement entered into with Genpharm Services FZ-ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 388 |
| LLC Global Anti-Bribery and Corruption Policy our Global Anti-Bribery and Corruption Policy GloBE Rules model rules in respect of Pillar Two GLPs good laboratory practices gMG generalized myasthenia gravis Greater China Mainland China, Hong Kong, Taiwan and Macau Group argenx SE together with its subsidiaries GSK GlaxoSmithKline plc Halozyme Halozyme Therapeutics, Inc. Handok Handok Inc. Handok Agreement an VYVGART commercial and distribution agreement entered into with Handok Hatch-Waxman Act the U.S. Drug Price Competition and Patent Term Restoration Act of 1984 HGF hepatocyte growth factor HHS U.S. Department of Health and Human Services HIPAA the U.S. federal Health Insurance Portability and Accountability Act of 1996 HITECH the Health Information Technology for Economic and Clinical Health Act of 2009 HRSA Health Resources and Services Administration IAVI International AIDS Vaccine Initiative IDMC Independent Data Monitoring Committee IFRS International Financial Reporting Standards, as issued by the International Accounting Standards Board, and as adopted by the European Union IgA Immunoglobulin A IgD Immunoglobulin D IgG Immunoglobulin G IgM Immunoglobulin M IIP Immunology innovation program IL-22R interleukin-22 receptor IMM irreversible morbidity or mortality IMNM immune-mediated necrotizing myopathy IND investigational new drug ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 389 |
| IQVIA IQVIA LTD IRA Inflation Reduction Act IRB institutional review board I-RODS Inflammatory Rasch-built Overall Disability Scale ISMS Information Security and Management System ISRs injection site reactions ISTs immunosuppressive therapies ITC Belgian Income Tax Code ITP immune thrombocytopenia IV intravenous IVIg intravenous IgG IWG International Working Group Janssen Janssen Pharmaceuticals, Inc. Johnson & Johnson Johson & Johnson Innovation, Inc. KPI key performance indicator LEI European legal entity identifier number LEO Pharma Pharma LEO Pharma A/S LEO Pharma Collaboration Agreement collaboration agreement entered into with LEO Pharma A/S LN lupus nephritis Lonza Lonza Sales AG LUMC Leiden University Medical Center Lundbeck H Lundbeck A/S MAA marketing authorization application mAb monoclonal antibody MADs multiple ascending doses Mainland China mainland China MAR Regulation (EU) No 596/2014 of the European Parliament and of the Council of April 2014 on market abuse (market abuse regulation) and repealing Directive 2003/6/EC European Parliament and of the Council and Commission Directives 2003/ 124/EC, 2003/EC and 2004/72/EC, and the rules and regulations promulgated pursuant thereto MBA Master’s of Business Administration Medison Medison Pharma Ltd. ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 390 |
| Medison Agreement exclusive distribution agreement entered into with Medison Pharma Ltd. to commercialize efgartigimod in Israel Medison Multi-Regional Agreement multi-regional agreement entered into with Medison Pharma Ltd. to commercialize efgartigimod in 14 countries MET mesenchymal-epithelial transition factor MG myasthenia gravis MG-ADL Myasthenia Gravis Activities of Daily Living MHLW Ministry of Health, Labour and Welfare MHRA Medicines and Healthcare products Regulatory Agency MMN multifocal motor neuropathy MN membranous nephropathy MRC QA Medical Regulatory and Clinical QA MSE minimal symptom expression Multi-Product License a non-exclusive multi-product in-license agreement with Lonza MuSK muscle-specific kinase Myositis idiopathic inflammatory myopathies Nasdaq the Nasdaq Global Select Market Nasdaq Listing Rules the listing rules of the Nasdaq Global Market NDA new drug application NEO named executive officer NFRD Directive 2014/95/EU of the European Parliament and the Council of 22 October 2014 amending Directive 2013/34/EU as regards disclosure of non-financial and diversity information by certain large undertakings and groups NHI National Health Insurance NHSA National Healthcare Security Administration NK natural killer NMJ neuro muscular junction Non-FAMP Non-Federal Average Manufacturer Price NRDL national reimbursement drug list NYU New York University NYU and LUMC Agreement collaboration and exclusive license agreements with NYU Langone Health and LUMC OCI other comprehensive income OFPs organizations for financing pensions ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 391 |
| OIG the Office of Inspector General OLE open-label extension OncoVerity OncoVerity, Inc. OpEx operating expenditure PAA pre-approval access program PBM pharmacy benefit managers PC-POTS Postural Orthostatic Tachycardia Syndrome Post-COVID-19 PD pharmacodynamic PDAI pemphigus disease area index PDUFA Prescription Drug User Fee Act PF pemphigus foliaceous PFIC passive foreign investment company Pharmaceutical and Medical Devices Act the Act on Securing Quality, Efficacy and Safety of Pharmaceuticals and Medical Devices PHSA the U.S. Public Health Service Act PIL Code Belgian Code of private international law Pillar Two the project, worked on by the OECD in recent years, aimed at reforming the international tax system by, among other matters ensuring large multinational enterprises pay a minimum level of tax in each of the jurisdictions in which they operate Pillar Two Directive Directive (EU) 2022/2523 on ensuring a global minimum level of taxation for multinational enterprise groups and large-scale domestic groups in the Union PIP pediatric investigation plan PK pharmacokinetic PMDA Pharmaceuticals and Medical Devices Agency (Japan) pMN primary MN POC proof-of-concept POTELLIGENT® License Agreements non-exclusive license agreements for POTELLIGENT® CHOK1SV with BioWa and Lonza PREA Pediatric Research Equity Act of 2003, as amended PRR partial renal response PSU performance share unit PV pemphigus vulgaris PVAS pemphigus vulgaris activity score QA quality assurance ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 392 |
| QMG quantitative myasthenia gravis RDL Reimbursable Drug List Relevant Regulatory Authorities the MHRA, EMA, FDA, MHLW REMS risk evaluation and mitigation strategy rHuPH20 recombinant human hyaluronidase PH20 Roche F. Hoffman-La Roche AG RSUs restricted stock units sBLA supplemental Biologics License Application SC subcutaneous SC subcutaneous SEC the U.S. Securities and Exchange Commission SEC Climate Rules final rules adopted by the SEC on March 6, 2024 aimed at enhancing and standardizing climate-related disclosures related to climate-related risks, Scope 1 and Scope 2 greenhouse gas emissions and climate-relateed financial metrics Securities Act the U.S. Securities Act of 1933, as amended Shire Shire AG, now known as Shire International GmbH Shire Collaboration Agreement collaboration agreement entered into with Shire AG, now known as Shire International GmbH SjD Sjögren’s disease SLE systemic lupus erythematosus Sopartec Sopartec S.A. System Lonza Sales AG’s proprietary glutamine synthetase gene expression system known as GS Xceed™ Targacept Targacept Inc. Taxonomy Environmental Objectives the six objectives included in the EU Taxonomy Regulation, being: (i) climate change mitigation, (ii) climate change adaption, (iii) sustainable use of protection of water and marine resources, (iv) transition to a circular economy, (v) pollution prevention, and (vi) protection and restoration of biodiversity and ecosystems TCA trade and cooperation agreement between the European Union and the United Kingdom formally applicable since May 1, 2021 TEAE treatment emergent adverse events TED Thyroid eye disease TGF-β transforming growth factor beta TIS total improvement score ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 393 |
| Transparency Directive Directive 2004/109/EC of the European Parliament and of the Council of December 15, 2004, on the harmonization of transparency requirements in relation to information about issuers whose securities are admitted to trading on a regulated market and amending Directive 2001/34/EC and the rules and regulations promulgated pursuant thereto, as amended by various directives including 2013/50/EU U.S. the United States of America U.S. Tax Treaty Convention between the Netherlands and the U.S. for the avoidance of Double Taxation and the Prevention of Fiscal Evasion with respect to Taxes and Income, dated December 18, 1992 as amended by the protocol of March 8, 2004 UCHealth University of Colorado Health UCL Université Catholique de Louvain UK the United Kingdom UK GDPR legal framework adopted by the United Kingdom substantially equivalent to the Regulation (EU) 2016/679 of the European Parliament and of the Council of April 27, 2016, on the protection of natural persons with regard to the processing of personal data and on the free movement of such data UPCR urine protein creatinine ratio USPTO the United States Patent and Trademark Office UT Agreement an exclusive in-license with the Board of Regents of the University of Texas System UT BoR the Board of Regents of the University of Texas System UT Southwestern University of Texas Southwestern Medical Center VIB VIB vzw VIB Agreement collaboration agreement entered into with VIB V-regions antibody variable regions VYVDURA VYVDURA® VYVGART VYVGART® VYVGART SC VYVGART subcutaneous VYVGART Approved Countries (i) U.S., Japan, UK, Mainland China, Canada, Israel and all 27 EU Member States plus Iceland, Norway and Liechtenstein for VYVGART for gMG, and (ii) the U.S. (as VYVGART HYTRULO), Japan (as VYVDURA), the UK and all 27 EU Member States plus Iceland, Norway and Liechtenstein for VYVGART SC VYVGART HYTRULO VYVGART HYTRULO™ we, us or our argenx SE together with its wholly owned subsidiaries and, as applicable, its former wholly owned subsidiaries Zai Lab Zai Lab Ltd ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 394 |
| Zai Lab Agreement collaboration agreement with Zai Lab Ltd, relating to an exclusive out-license for the development and commercialization of efgartigimod in Greater China Zai Lab Payments $75.0 million upfront payment under the collaboration with Zai Lab Ltd in the form of 568,182 newly issued Zai Lab shares calculated at a price of $132.0 per share, a $75.0 million guaranteed non-creditable, non-refundable development cost-sharing payment and a $25.0 million milestone payment in connection with FDA approval of VYVGART ar Gr g oup enx Factors Risk Go Corporate vernance Capital Share Financial Review Statements Financial Non-Financial Information argenx Annual Report 2023 Definitions | 395 |
| Contact us via argenx.com/contact-us You can find the annual report 2023 online at reports.argenx.com/2023 |