
KOL对GA治疗前景和未满足需求的见解18 ""尽管FDA批准了GA的第一种治疗方法,但仍有重大未满足需求。重要的是我们减少治疗的频率,治疗必须通过玻璃体内注射到眼睛。此外,治疗地图状萎缩同时防止脉络膜新生血管形成是另一个重要的未满足需求。 Elias Reichel,医学博士 眼科教授 塔夫茨大学医学院由Akari主办的关键意见领袖活动讨论了GA的诊断、治疗和重大未满足需求https://www.example.com/event/akari—event/

1. 补体C5抑制以减缓GA进展频繁的针头注射到眼睛后部,一个来源的恐惧,不适和破坏的患者3;可能每年注射4次或更少的PAS—诺马可潘LTB 4抑制可以防止VEGF—A过表达,VEGF—A是威胁视力的CNV的关键驱动因素,4与批准用于GA治疗的仅补体抑制剂相关的安全性风险(用VEGF抑制剂治疗)补体C3和C5抑制剂减缓GA病变进展的功效是众所周知的1,2PAS—nomacopan在GA 2中. 更少的针头注射到眼睛3. 抑制LTB4可降低CNV PAS的风险—Nomacopan可提供3个关键益处:补体抑制、更少剂量和抑制LTB4以解决CNV风险 19参考文献1. Liao DS,et al.,补体C3抑制剂pegcetacoplan治疗继发于年龄相关性黄斑变性的地图状萎缩—一项随机II期试验。 眼科学2019;127:586—195。2. Jaffe GJ等人,C5抑制剂avaccapapad治疗年龄相关性黄斑变性引起的地图状萎缩—一项随机关键性2/3期试验。 眼科学2021;128:576—586。3. McClard CK,et al. Assessment Life Impact of Treatment by Intrautral Injection(VILITII):Development of a patient reported measure to assess reported measure for repeat injection of repeated vinjection). BMJ开放眼。2021;6(1):e000669。4. Sasaki F,et al.,在鼠湿型AMD模型中,白三烯B4促进新生血管形成和巨噬细胞募集. JCI Insight 2018;3:e96902。

长效PAS—Nomacopan有可能将玻璃体内给药间隔延长至3个月或更长时间110 100 1,000 10,000 0 20 40 60 80 100 120 PAS—玻璃体浓度(µ g/mL)时间(天)0.05mL PAS600—诺马可潘(20 mg/mL)0.05mL PAS600—诺马可潘(60 mg/mL)长效PAS—诺玛可每年向眼睛内注射4次或更少的潜力·PK/PD数据显示PAS—诺玛可延长玻璃体内注射后在眼睛内的半衰期(7.4—8.4天),提示给药间隔可能为3个月或更长1参考文献:1.韦斯顿—戴维斯,W.,等.开发长效PAS—诺马可潘用于治疗GA和其它视网膜疾病.海报展示ARVO,2022.家兔中的20个观察数据预测浓度0.1mL PAS—nomacopan(60mg/mL)50 μ L PAS—nomacopan 20mg/mL 50 μ L PAS—nomacopan 60mg/mL来源:PAS—来自内部研究的nomacopan数据视网膜生物利用度视网膜0 5 10 15 20 25 30 35 40 45 50 0 7 14 21 28%玻璃体浓度(平均值± SEM)注射后天数Aflibercept PAS—nomacopan

PAS—Nomacopan与抗VEGF抗体一样有效地降低临床前模型中VEGF水平 (p g/m l)S alin e noma PA S—n o m a an t i—V E G F H e a l t h y CT0100200300p = 0.044p = 0.048PAS—诺马可潘对重度色素层炎的标准临床前模型中VEGF水平的影响21在重度色素层炎的临床前模型中,长效PAS—诺马可潘(单次IVI)VEGF水平降低(VEGF—A是CNV的关键驱动因素)与抗VEGF抗体治疗1一样有效,2LTB4促进湿性年龄相关性黄斑变性临床前模型中激光诱导的CNV 3玻璃体内VEGF浓度(pg/ml)Nomacopan盐水抗VEGF健康对照PAS—Nomacopan参考文献1. Eskandarpour M等人,实验性自身免疫性色素层炎和人类视网膜组织中的白三烯B4及其受体—视网膜Th17细胞的临床严重程度和LTB 4依赖性。 我是J Pathol。2021;191:320—334 Eskandapour M等人,免疫介导的后色素层炎视网膜血管炎和实验模型:白三烯(LT)B4—VEGF轴。 第2021章:10:396 3. Sasaki F,et al.,白三烯B4促进小鼠湿型AMD模型中新生血管形成和巨噬细胞募集. JCI Insight 2018;3:e96902

22谢谢你

Akari/Peak Bio Definitive Agreement/Merger of Equals 5 Akari Therapeutics and Peak Bio Announce Definitive Agreement to Merge as Equals Creating an Expanded Pipeline That Features a Novel Antibody Drug Conjugate (ADC) Toolkit Following closing, the combined company will have an expanded pipeline that contains multiple compelling assets spanning early and late development stages Key highlights of the merger include: o Peak Bio’s innovative antibody drug conjugate (ADC) toolkit with novel toxin and linker technology, expected to be the merged company’s lead asset: program includes a novel pre - clinical ADC candidate targeting TROP - 2 o Akari’s nomacopan, a bispecific recombinant inhibitor of complement C5 and leukotriene B4 (LTB4), in Phase 3 for pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA) o Akari’s long - acting version of nomacopan (PASylated - nomacopan) in the final stages of pre - clinical development for geographic atrophy (GA) with potential to address significant unmet needs o Peak Bio’s Phase 2 - ready neutrophil elastase inhibitor (NEI) targeting alpha - 1 antitrypsin deficiency (AATD); program licensed from Bayer Healthcare and is a 5th generation neutrophil elastase inhibitor (NEI) targeting the inflammatory aspects of AATD, a rare condition o Strategic emphasis on business development and licensing with broad potential impact on patients o Proven leadership with extensive strategic and operational experience

6 AKARI OVERVIEW
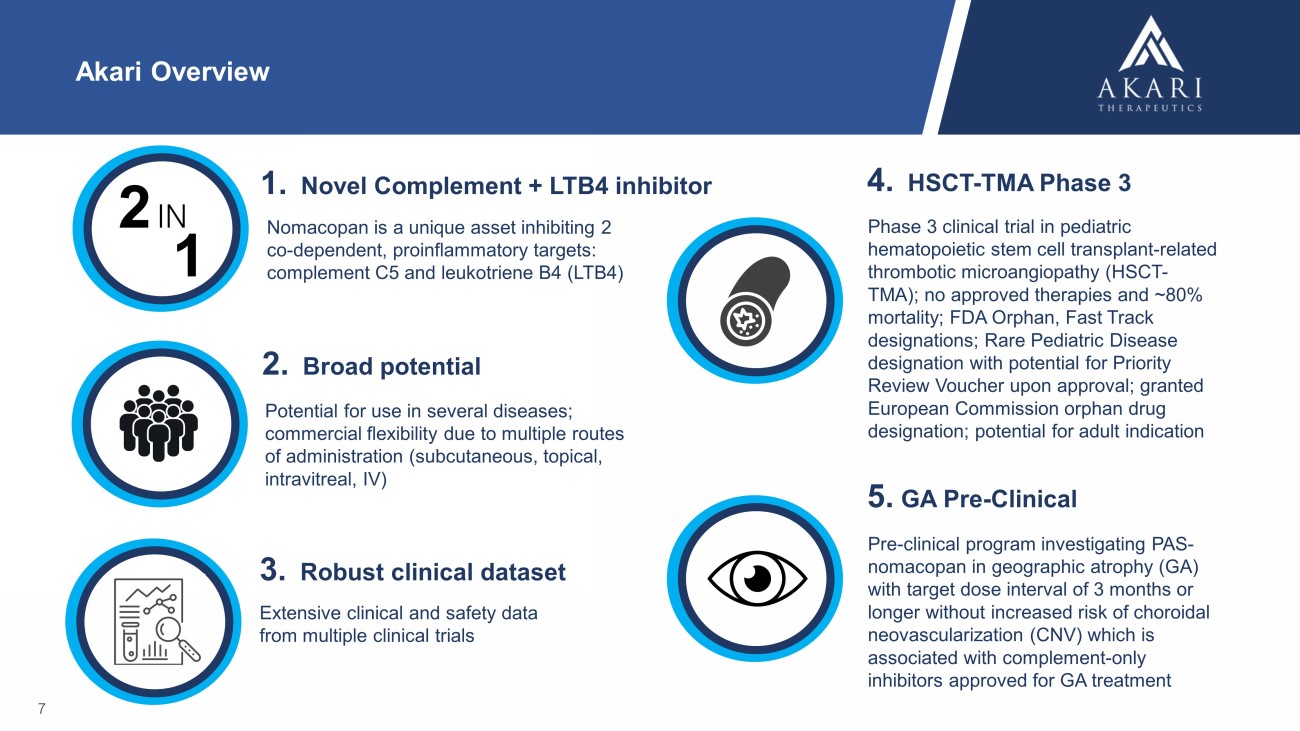
Akari Overview Nomacopan is a unique asset inhibiting 2 co - dependent, proinflammatory targets: complement C5 and leukotriene B4 (LTB4) 7 Potential for use in several diseases; commercial flexibility due to multiple routes of administration (subcutaneous, topical, intravitreal, IV) 1. Novel Complement + LTB4 inhibitor 2. Broad potential Extensive clinical and safety data from multiple clinical trials 3. Robust clinical dataset 2 IN 1 Phase 3 clinical trial in pediatric hematopoietic stem cell transplant - related thrombotic microangiopathy (HSCT - TMA); no approved therapies and ~80% mortality; FDA Orphan, Fast Track designations; Rare Pediatric Disease designation with potential for Priority Review Voucher upon approval; granted European Commission orphan drug designation; potential for adult indication 4. HSCT - TMA Phase 3 5. GA Pre - Clinical Pre - clinical program investigating PAS - nomacopan in geographic atrophy (GA) with target dose interval of 3 months or longer without increased risk of choroidal neovascularization (CNV) which is associated with complement - only inhibitors approved for GA treatment
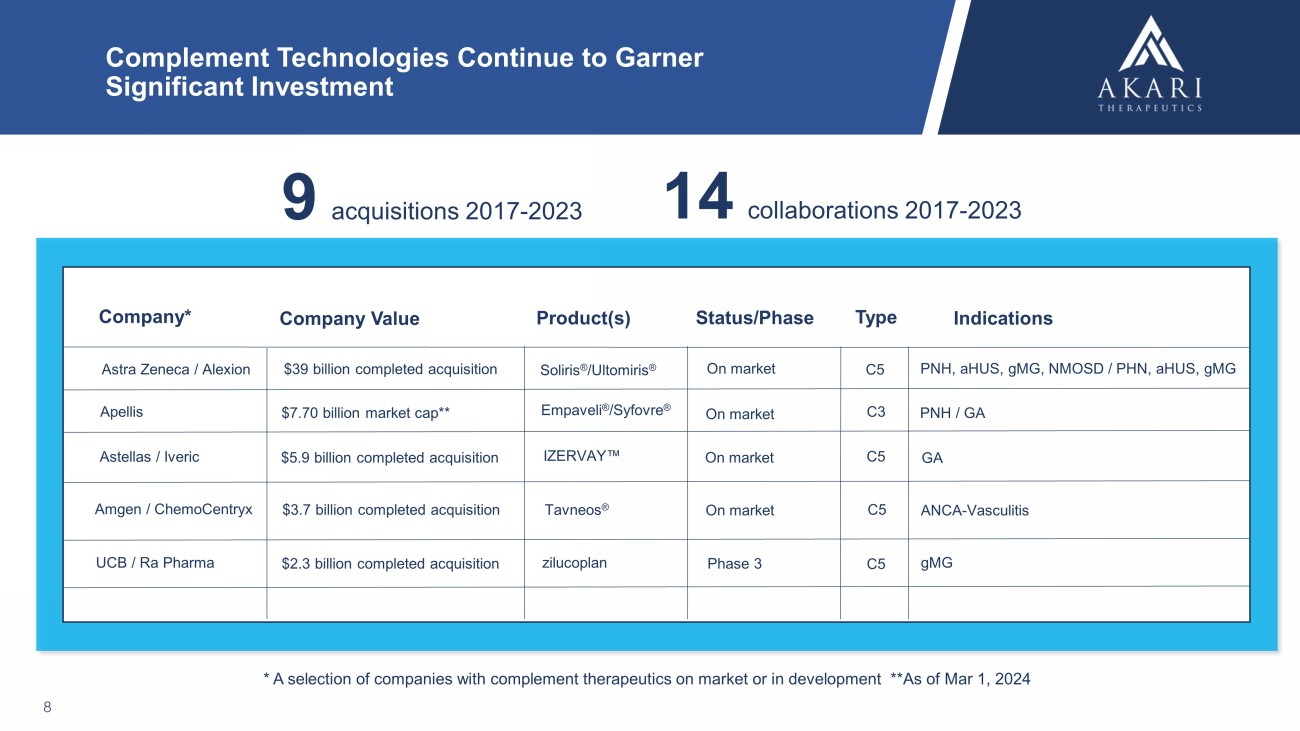
Complement Technologies Continue to Garner Significant Investment 8 Astra Zeneca / Alexion Company* Company Value Status/Phase Indications $39 billion completed acquisition Soliris ® / Ultomiris ® PNH, aHUS , gMG , NMOSD / PHN, aHUS , gMG Tavneos ® Amgen / ChemoCentryx ANCA - Vasculitis $7.70 billion market cap** Empaveli ® / Syfovre ® PNH / GA * A selection of companies with complement therapeutics on market or in development **As of Mar 1, 2024 Product(s) Type $3.7 billion completed acquisition C5 On market Apellis C3 On market Astellas / Iveric $5.9 billion completed acquisition C5 GA On market IZERVAY Œ C5 On market UCB / Ra Pharma $2.3 billion completed acquisition zilucoplan Phase 3 C5 gMG 9 acquisitions 2017 - 2023 14 collaborations 2017 - 2023
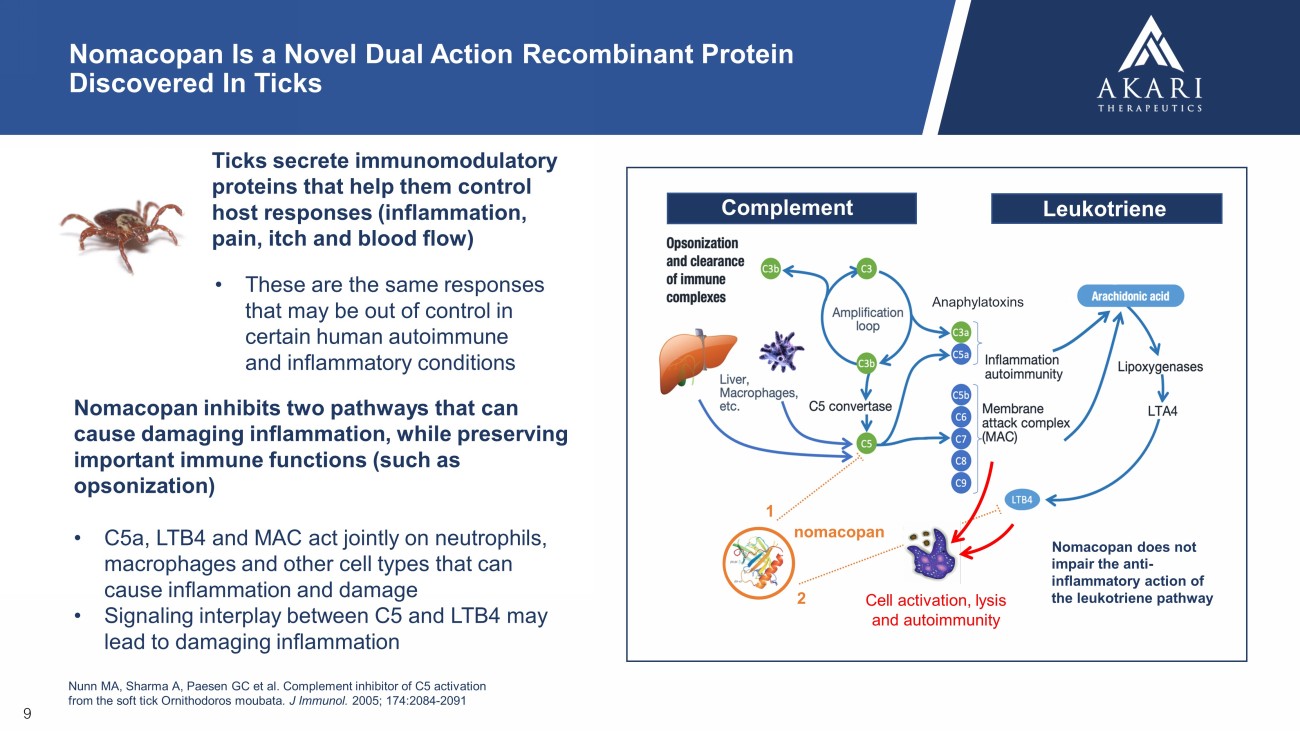
Nomacopan inhibits two pathways that can cause damaging inflammation, while preserving important immune functions (such as opsonization) • C5a, LTB4 and MAC act jointly on neutrophils, macrophages and other cell types that can cause inflammation and damage • Signaling interplay between C5 and LTB4 may lead to damaging inflammation Nunn MA, Sharma A, Paesen GC et al. Complement inhibitor of C5 activation from the soft tick Ornithodoros moubata . J Immunol. 2005; 174:2084 - 2091 Cell activation, lysis and autoimmunity nomacopan 1 2 Complement Leukotriene Anaphylatoxins Nomacopan does not impair the anti - inflammatory action of the leukotriene pathway 9 Nomacopan Is a Novel Dual Action Recombinant Protein Discovered In Ticks T icks secrete immunomodulatory proteins that help them control host responses (inflammation, pain, itch and blood flow) • These are the same responses that may be out of control in certain human autoimmune and inflammatory conditions
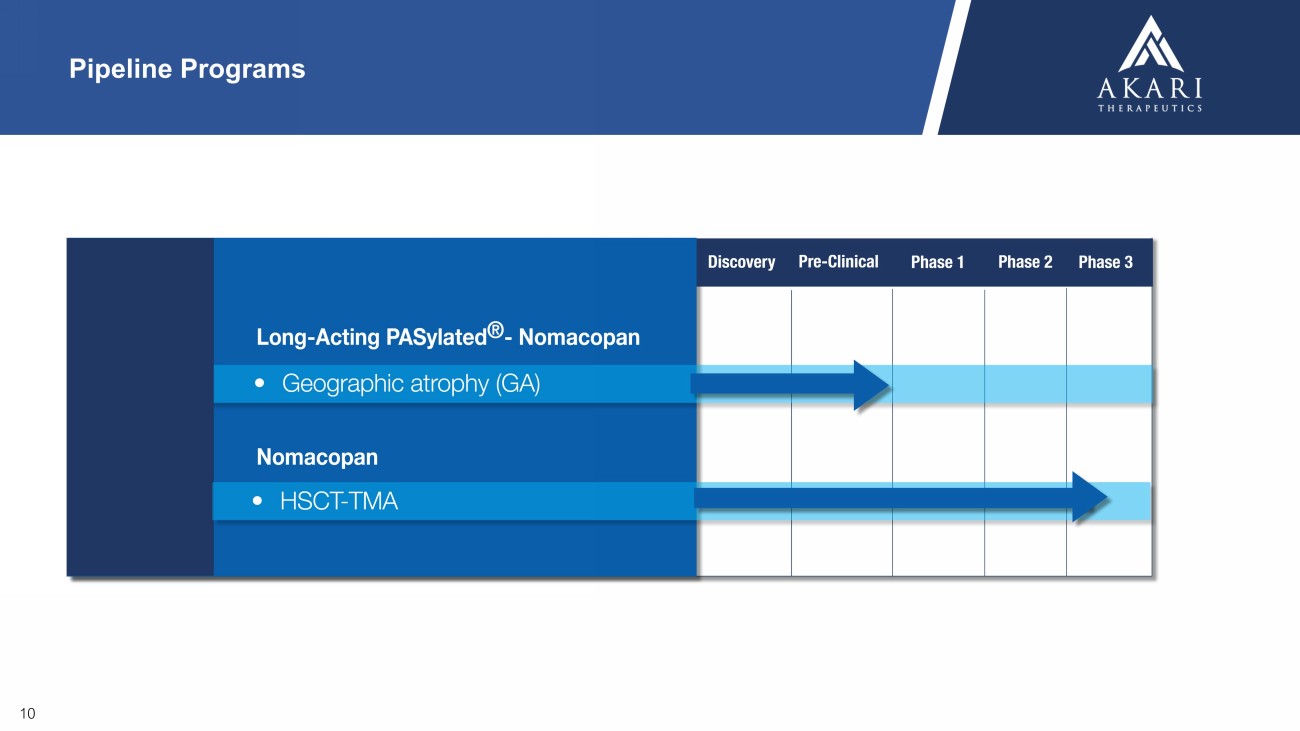
10 Pipeline Programs
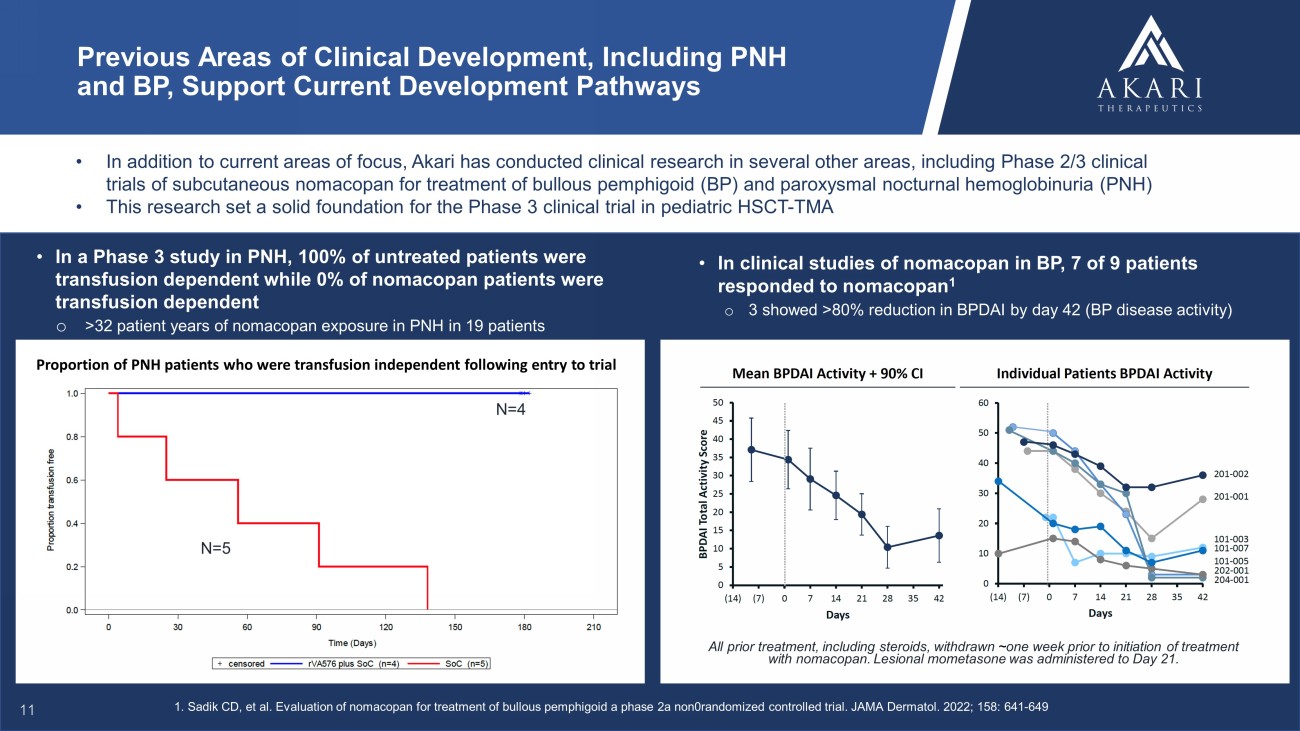
• In addition to current areas of focus, Akari has conducted clinical research in several other areas, including Phase 2/3 clin ica l trials of subcutaneous nomacopan for treatment of bullous pemphigoid (BP) and paroxysmal nocturnal hemoglobinuria (PNH) • This research set a solid foundation for the Phase 3 clinical trial in pediatric HSCT - TMA Previous Areas of Clinical Development, Including PNH and BP, Support Current Development Pathways • In clinical studies of nomacopan in BP, 7 of 9 patients responded to nomacopan 1 o 3 showed >80% reduction in BPDAI by day 42 (BP disease activity) All prior treatment, including steroids, withdrawn ~ one week prior to initiation of treatment with nomacopan. Lesional mometasone was administered to Day 21. • In a Phase 3 study in PNH, 100% of untreated patients were transfusion dependent while 0% of nomacopan patients were transfusion dependent o >32 patient years of nomacopan exposure in PNH in 19 patients Proportion of PNH patients who were transfusion independent following entry to trial 1. Sadik CD, et al. Evaluation of nomacopan for treatment of bullous pemphigoid a phase 2a non0randomized controlled trial. JAMA Dermatol. 2022; 158: 641 - 649 N=4 N=5 11

12 AKARI DEVELOPMENT PROGRAMS

13 NOMACOPAN IN HSCT - TMA
14 • HSCT - TMA is a rare but serious complication of HSCT involving complement activation, inflammation, tissue hypoxia and blood clots, leading to progressive organ damage and death • Graft versus host disease is commonly present in patients with severe HSCT - TMA 1 • Mortality is 80% across adults and children (severe) 2 • No approved treatment options Nomacopan May Be the First Treatment for HSCT - TMA, a Condition with Mortality Up to 80% 1. Complement C5 inhibition efficacy Nomacopan clinical trials are establishing a simple, fixed dose in children; ease of dosing at home or in hospital for adults LTB4 is often elevated in patients with GVHD and nomacopan inhibition of LTB4 may slow GVHD progression 4 1. Jodele S, et al. Complement blockade for TA - TMA: lessons learned from a large pediatric cohort treated with eculizumab. Blood . 2020;135(13):1049 - 1057. 2. Rosenthal J. Hematopoietic cell transplantation - associated thrombotic microangiopathy: a review of pathophysiology, diagnosis, a nd treatment. J Blood Med. 2016;7:181 - 186. 3. Schols S, Nunn MA, Mackie I et al. Succesful treatment of a PNH patient non - responsive to eculizumab with novel complement C5 inhibitor covers (nomacopan). Br J Hematol . 2020; 188: 332 - 340. 4. Takatsuka H, et al. Predicting the severity of intestinal graft - versus - host disease from leukotriene B4 levels after bone marrow transpla ntation. Bone Marrow Transplant. 2000;26(12):1313 - 1316. Nomacopan C5 inhibition supported by clinical PNH research 3 Nomacopan in HSCT - TMA 2. Simple, fixed dosing 3. Rapid onset & offset of action 4. LTB4 inhibition may slow GVHD progression Rapid onset/offset of action allows complement re - activation when needed
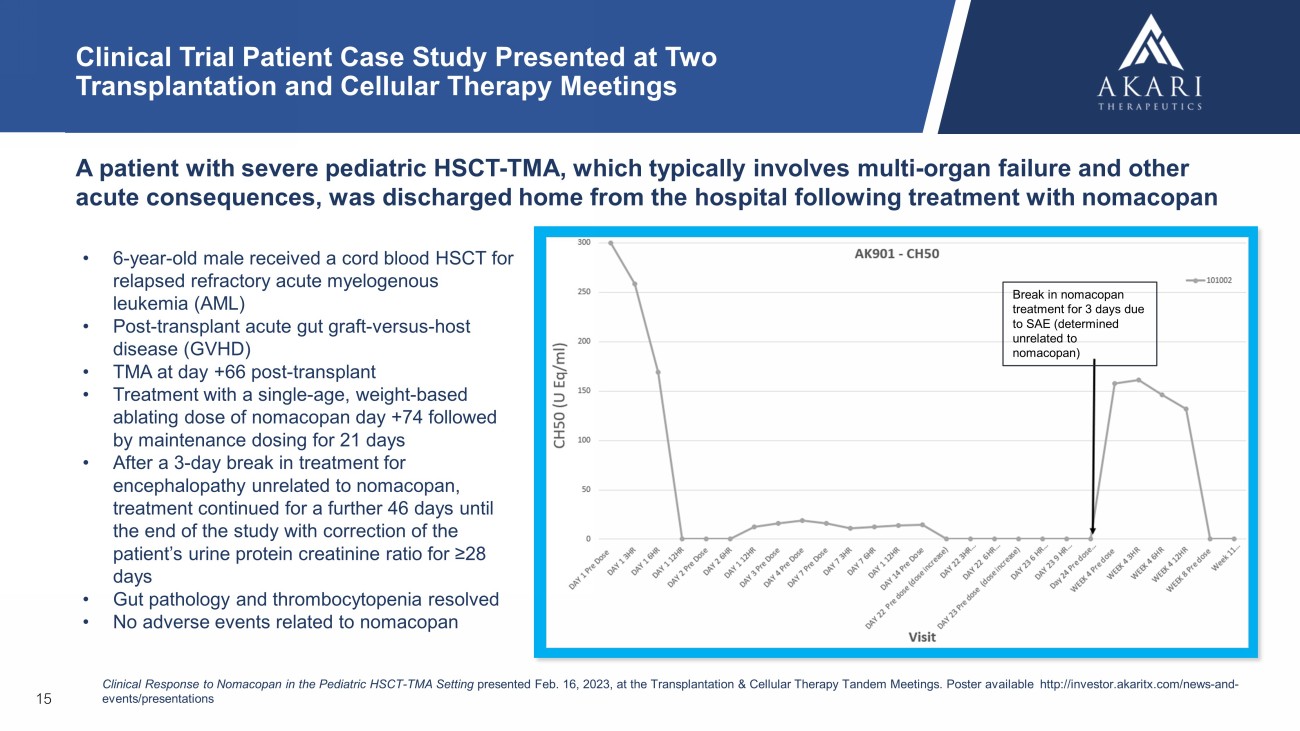
15 Clinical Trial Patient Case Study Presented at Two Transplantation and Cellular Therapy Meetings A patient with severe pediatric HSCT - TMA, which typically involves multi - organ failure and other acute consequences, was discharged home from the hospital following treatment with nomacopan • 6 - year - old male received a cord blood HSCT for relapsed refractory acute myelogenous leukemia (AML) • Post - transplant acute gut graft - versus - host disease (GVHD) • TMA at d ay +66 post - transplant • T reatment with a single - age, weight - based ablating dose of nomacopan day +74 followed by maintenance dosing for 21 days • After a 3 - day break in treatment for encephalopathy unrelated to nomacopan, treatment continued for a further 46 days until the end of the study with correction of the patient’s urine protein creatinine ratio for ≥28 days • Gut pathology and thrombocytopenia resolved • No adverse events related to nomacopan Clinical Response to Nomacopan in the Pediatric HSCT - TMA Setting presented Feb. 16, 2023, at the Transplantation & Cellular Therapy Tandem Meetings. P oster available http:// investor.akaritx.com /news - and - events/presentations Break in nomacopan treatment for 3 days due to SAE (determined unrelated to nomacopan)

16 GEOGRAPHIC ATROPHY (GA)

17 • Geographic atrophy (GA) manifests as a chronic progressive degeneration of the macula, which occurs during late - stage dry age - related macular degeneration (dAMD) and can lead to irreversible vision loss • Approximately 5 million people worldwide are affected, 1,2 with nearly 1 million in the U.S. 3 • The first treatments for GA have been approved by the FDA in 2023 References 1. Wong WL, et al. Global prevalence of age - related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta - analysis. Lancet Glob Health. 2014;2(2):e106 - e116. 2. Rudnicka AR, et al. Age and gender variations in age - related macular degeneration prevalence in populations of European ancestry: a meta - analysis. Ophthalmology. 2012;119(3):571 - 580. 3. Friedman DS, et al. Prevalence of age - related macular degeneration in the United States [published correction appears in Arch Ophthalmol . 2011 Sep;129(9):1188]. Arch Ophthalmol . 2004;122(4):564 - 572. Geographic Atrophy (GA)

KOL Insights on GA Treatment Landscape and Unmet Needs 18 “ ” Despite FDA approvals of the first treatments for GA, there are still significant unmet needs. It’s important that we reduce the frequency of therapy, which must be administered through intravitreal injection into the eye. In addition, treating geographic atrophy while preventing choroidal neovascularization from developing is another important unmet need. Elias Reichel, M.D. Professor of Ophthalmology Tufts University School of Medicine A key opinion leader event hosted by Akari discussed GA diagnosis, treatment, and significant unmet needs https:// lifescievents.com /event/ akari - event/

1. Complement C5 inhibition to slow GA progression Frequent needle injections into the back of the eye, a source of fear, discomfort and disruption for patients 3 ; potential for 4 or fewer injections with PAS - nomacopan each year LTB4 inhibition may prevent VEGF - A overexpression, a key driver of sight - threatening CNV, 4 a safety risk (treated with VEGF inhibitors) associated with complement - only inhibitors approved for GA treatment Efficacy of complement C3 and C5 inhibition slowing progression of GA lesions is well understood 1,2 PAS - nomacopan in GA 2. Fewer needle injections into the eye 3. LTB4 inhibition may reduce risk of CNV PAS - Nomacopan May Provide 3 Key Benefits: Complement Inhibition, Fewer Doses & LTB4 Inhibition to Address CNV Risk 19 References 1. Liao DS, et al., Complement C3 inhibitor pegcetacoplan for geographic atrophy secondary to age - related macular degeneration - a randomised phase 2 trial. Opthalmology 2019; 127: 586 - 195. 2. Jaffe GJ, et al., C5 inhibitor avacincaptad peg for geographic atrophy due to age - related macular degeneration - a randomised pivotal phase 2/3 trial. Ophthalmology 2021; 128: 576 - 586. 3. McClard CK, et al. Questionnaire to Assess Life Impact of Treatment by Intravitreal Injections (QUALITII): Development of a p ati ent - reported measure to assess treatment burden of repeat intravitreal injections. BMJ Open Ophthalmol . 2021;6(1):e000669. 4. Sasaki F, et al., Leukotriene B4 promotes neovascularisation and macrophage recruitment in murine wet - type AMD models. JCI Insight 2018; 3: e96902.
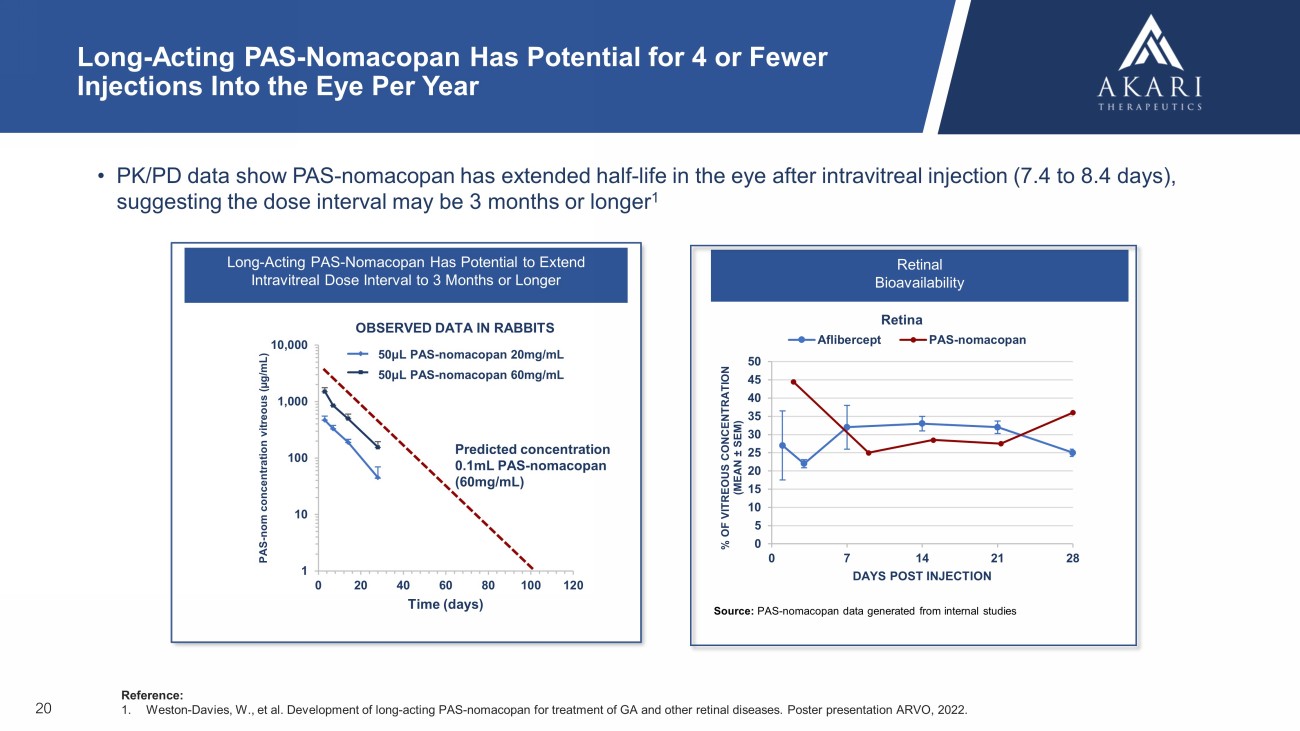
Long - Acting PAS - Nomacopan Has Potential to Extend Intravitreal Dose Interval to 3 Months or Longer 1 10 100 1,000 10,000 0 20 40 60 80 100 120 PAS - nom concentration vitreous ( µg/mL) Time (days) 0.05mL PAS600-nomacopan (20 mg/mL) 0.05mL PAS600-nomacopan (60 mg/mL) Long - Acting PAS - Nomacopan Has Potential for 4 or Fewer Injections Into the Eye Per Year • PK/PD data show PAS - nomacopan has extended half - life in the eye after intravitreal injection (7.4 to 8.4 days), suggesting the dose interval may be 3 months or longer 1 Reference: 1. Weston - Davies, W., et al. Development of long - acting PAS - nomacopan for treatment of GA and other retinal diseases. Poster presen tation ARVO, 2022. 20 OBSERVED DATA IN RABBITS Predicted concentration 0.1mL PAS - nomacopan (60mg/mL) 50μL PAS - nomacopan 20mg/mL 50μL PAS - nomacopan 60mg/mL Source: PAS - nomacopan data generated from internal studies Retinal Bioavailability Retina 0 5 10 15 20 25 30 35 40 45 50 0 7 14 21 28 % OF VITREOUS CONCENTRATION (MEAN ± SEM) DAYS POST INJECTION Aflibercept PAS-nomacopan
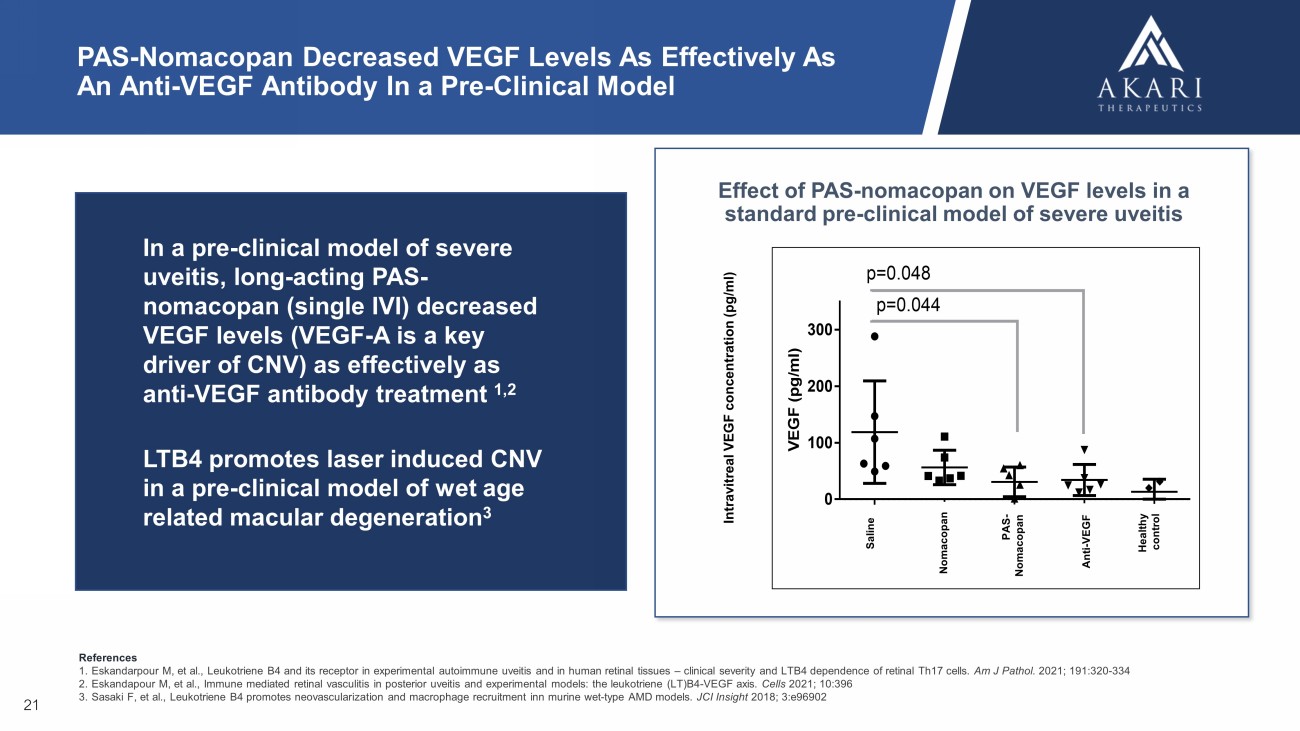
PAS - Nomacopan Decreased VEGF Levels As Effectively As An Anti - VEGF Antibody In a Pre - Clinical Model V E G F ( p g / m l ) S a l i n e n o m a P A S - n o m a a n t i - V E G F H e a l t h y c t 0 100 200 300 p=0.044 p=0.048 Effect of PAS - nomacopan on VEGF levels in a standard pre - clinical model of severe uveitis 21 In a pre - clinical model of severe uveitis, long - acting PAS - nomacopan (single IVI) decreased VEGF levels (VEGF - A is a key driver of CNV) as effectively as anti - VEGF antibody treatment 1,2 LTB4 promotes laser induced CNV in a pre - clinical model of wet age related macular degeneration 3 Intravitreal VEGF concentration ( pg /ml) Nomacopan Saline Anti - VEGF Healthy control PAS - Nomacopan References 1. Eskandarpour M, et al., Leukotriene B4 and its receptor in experimental autoimmune uveitis and in human retinal tissues – clinical severity and LTB4 dependence of retinal Th17 cells. Am J Pathol . 2021; 191:320 - 334 2. Eskandapour M, et al., Immune mediated retinal vasculitis in posterior uveitis and experimental models: the leukotriene (LT)B4 - VEGF axis. Cells 2021; 10:396 3. Sasaki F, et al., Leukotriene B4 promotes neovascularization and macrophage recruitment inn murine wet - type AMD models. JCI Insight 2018; 3:e96902

22 THANK YOU